DISCUSSION
Dox is considered as one of the most potent anticancer agents. Its therapeutic application is limited due to its deleterious effects on normal tissues, such as the liver and heart (Ibrahim et al., 2010; Wang et al., 2012). However, the mechanisms of Dox-mediated cytotoxicity in normal tissues and cancer cells are different (Wang et al., 2012). In its mechanisms of action, Dox may act through DNA intercalation, membrane function alteration, and ROS formation (King and Perry, 2001). Dox is considerably metabolized in the liver (King and Perry, 2001); thereby, the liver is one of the organs that are mostly affected by Dox toxicity.
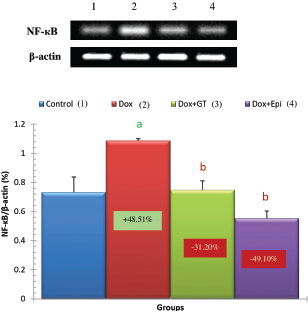 | Figure 5. Effect of green tea aqueous extract and epicatechin on liver NF-κB mRNA expression relative to β-actin in Dox-administered rats. a,bSignificant difference in comparison with the corresponding control and Dox-administered groups, respectively, at p < 0.05. Percentage changes were calculated in relation to the normal control and Dox-administered groups, respectively.
[Click here to view] |
It is worth mentioning from many previous publications that Dox is considered as the most toxic anthracyclines and causes weight loss (Danz et al., 2009; Panjrath et al., 2007; Sayed-Ahmed et al., 2010). For this reason, overdosage and long-term administration of this drug causes toxicity and death.
The main toxic and deleterious effects on hepatocytes include disruption of electron transport and oxidative stress and arrest cell cycle of hepatocytes (Kassner et al., 2008; Zhao et al., 2012). To reduce the toxic and side effects of Dox, various strategies were reported (EL-Hak et al., 2018; Jabłońska-Trypuć et al., 2018). The newly developed chemotherapeutic drugs often cause many side effects that restrict their use which are mostly oxidative stress-dependent (Ahmed and Abdella, 2010). Therefore, recent studies hypothesized that the combination of the chemotherapeutic drug such as Dox together with a potent natural antioxidant may be the appropriate approach to mitigate the toxic side effects of this kind of drugs (Ahmed and Abdella, 2010; Injac et al., 2008).
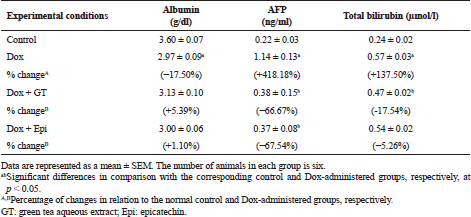
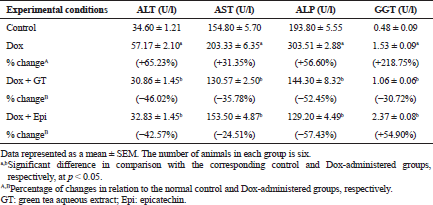
The present study revealed that Dox injected weekly at dose 4 mg Dox/kg b.w. through intraperitoneal route for 6 weeks induced hepatotoxicity which was biochemically manifested by a significant elevation of serum ALT, AST, ALP, and GGT activities and bilirubin level in addition to a significant lowering in serum level of albumin. These changes run parallel with Ahmed et al. (2013) and Injac et al. (2008) who attributed the elevation in the serum enzyme activities to their excess leakage from degenerated hepatocytes and damage in bile ductular cells membranes as a result of toxicity. The significant decrease in serum albumin level in Dox-administered rats is in accordance with EL-Maraghy et al. (2009) who attributed this change to alterations in protein and free amino acid metabolism and their synthesis in the injured hepatocytes and/or increased protein degradation. On the other hand, the elevation in the total bilirubin level in serum of Dox-administered rats may be due to blockage of bile canaliculi as a result of inflammatory cells infiltration, fibroblast proliferation and fibrosis in the portal areas, and/or may be owing to regurgitation of direct (conjugated) bilirubin from the necrotic liver cells to sinusoids (Ahmed, 2001; Deepa and Varalakshmi, 2003; Saad et al., 2001).
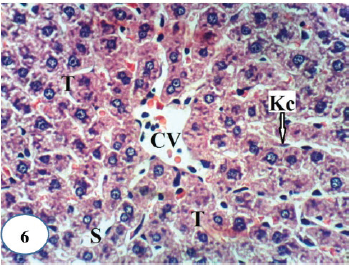 | Figure 6. A photomicrograph of liver section of normal rats showing a normal histological structure of hepatic lobule and depicting a CV, hepatic strands or trabeculae (T) with sinusoids (S) and Kupffer cells (Kc) in-between (H&E ×400).
[Click here to view] |
The previous deleterious biochemical alterations of liver biomarkers in serum of Dox-administered rats in the current study were accompanied with a remarkable increase in liver LPO and a significant suppression in the liver levels of non-enzymatic antioxidant (GSH) as well as enzymatic antioxidants (SOD, GPx, and GST). These results are in concurrence with those obtained by several authors (Abd El-Aziz et al., 2001; Ahmed et al., 2013; Balachandar et al., 2003; Kalender et al., 2005; Patel et al., 2010; Yagmurca et al., 2007) who stated that one of the most convincing hypotheses of hepatic injury due to Dox injection is the ability of the drug to produce excess free radicals and lipid peroxides and to suppress free radical scavenging capacity and antioxidant defensive mechanism.
Histopathological investigation of liver sections of Dox-administered rats in the present study supported the previous biochemical results. The liver of Dox-administered rats showed congested CVs, hyperemic sinusoids, Kupffer cell multiplication, strands of fibroblasts around the hepatocytes, cytoplasmic vacuolization of hepatocytes, fibrosis of hepatic capsule, and periductal fibroblastic proliferation around the bile ductules. These results are in concordance with Yagmurca et al. (2007) who reported destructive damage of hepatocytes, necrotic foci, blood congestion, and proliferation of bile canaliculi in Dox-supplemented rats.
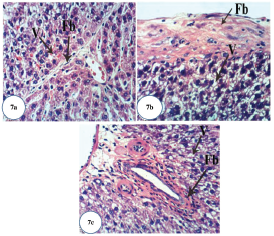 | Figure 7. Photomicrographs of liver section of Dox-administered rats showing: (a) strands of fibroblasts (Fb) around the hepatocytes and vacuolization (V) of hepatocytes (H&E ×400); (b) fibrosis of hepatic capsule and vacuolization (V) of hepatocytes (H&E ×400); and (c) fibroplasia of bile duct and vacuolization (V) of hepatocytes (H&E ×400).
[Click here to view] |
Antioxidants obtained from natural sources and plants represent a logical treatment strategy for therapy of liver diseases. In this regard, there are many plant-derived chemicals with powerful antioxidant properties that may serve as primary compounds for developing novel hepatoprotective drugs (Girish and Pradhan, 2012; Pradhan and Girish, 2006). Green tea and its constituting catechins are best known for their free radical scavenging capabilities, which have led to their evaluation in a number of diseases associated with exacerbated production of ROS, such as diabetes mellitus, cancer, neurodegenerative diseases, and cardiovascular disorders. Several epidemiological and meta-analysis studies as well as studies in animal models have depicted that green tea can afford protection against various types of cancers including breast, skin, lung, and prostate cancers (Mukhtar and Ahmad, 2000; Yang et al., 2002). The epicatechin as one of the major constituent of green tea is considered as one of the most potent antioxidant component of catechin (Ishige et al., 2001) due to its higher free radical scavenging activity (Kostyuk et al., 2004) and its greater bioavailability over other catechin components (Baba et al., 2001).
In conduction with the previous studies, the treatment of Dox-administered rats with green tea aqueous extract and epicatechin potentially alleviated the raised serum ALT, AST, and ALP enzyme activities and bilirubin level. The lowered serum albumin level was detectably increased in Dox-administered rats treated with green tea aqueous extract and epicatechin. These improvements in serum biomarkers of liver function were associated with potential amendment of liver integrity and architecture and amelioration of Dox-induced deleterious hispathological changes. These alterations are in concordance with the previously published report of Mandziuk et al. (2015) who depicted that Dox-induced inflammation, eosinophilic degeneration, and interstitial edematous changes were markedly reduced by green tea. The ameliorative effects of green tea aqueous extract and epicatechin may be attributed to the potentiation of the antioxidant defense system and suppression of ROS generation. It is important here to mention that the ALP activity was more decreased in the Dox-administered rats treated with green tea aqueous extract and epicatechin than the normal control. The decrease lower than the normal due to treatment with green tea may be attributed to the presence of epigallocatechin gallate and gallocatechin gallate, which have inhibitory effects on phosphatases (Okamoto et al., 2003) due to the presence of galloyl moiety in the structure. The epicatechin may also have inhibitory effects on ALP activity. The serum activity of GGT, responsible for extracellular GSH, was significantly increased as a result of treatment of Dox-administered rats with epicatechin. This up-regulation of GGT activity may be attributed in the light of suggestion of Chinta et al. (2006) who hypothesized that increased GGT activity may be an adaptive response to the loss of glutathione to conserve intracellular GSH content and results in a compensatory effect on mitochondrial complex I activity rather than in its inhibition and decrease following improvement of hepatobiliary function.
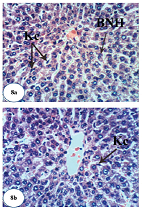 | Figure 8. Photomicrographs of liver sections of Dox-administered rats treated with green tea. (a and b) Marked recovery of normal structure except the presence of few Kupffer cells (Kc) activation and binucleation of hepatocytes (BNH) (H&E ×400).
[Click here to view] |
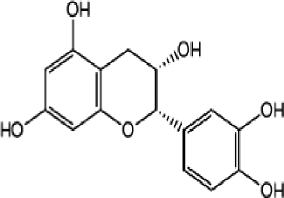
In the present study, the treatment of Dox-injected rats with green tea aqueous extract and epicatechin reduced liver LPO and increased the liver GSH level and GPx, GST, and SOD enzyme activities. These antioxidative features of green tea and its catechin have been reported in previous in vivo studies, which demonstrated that dietary intake of green tea catechins can improve total antioxidant capacity and can decrease the level malondialdehyde (MDA), as a biomarker of LPO, in the rat’s liver, blood, and brain (Skrzydlewska et al., 2000). In another study, Quine and Raghu (2005) showed that epicatechin supplementation at a dose of 15 and 30 mg/kg b.w. in diabetic rats produced a significant decrease in MDA levels and increase in GSH concentration and SOD, GPx, and catalase (CAT) activities in the liver. In the same regard, Rizvi et al. (2005) had revealed that epicatechin treatment produced an elevation in GSH content in red blood cells of both normal and type 2 diabetic subjects. Rizvi and Zaid (2001) have also stated that tea catechins (epicatechin is being one of the components) protect type 2 diabetic red blood cells from tert-butyl hydroperoxide-induced oxidative stress. Moreover, Cuevas et al. (2009) found that epicatechin produced a significant decrease in LPO and reactive oxygen species in amyloid-β-treated rats. Mohamed et al. (2011) reported that SOD, CAT, and GPx in the brain tissues in Dox-administered rats were normalized and the elevated level of MDA was decreased upon using epicatechin supplementation. The strong free radical scavenging activity of epicatechin might be due to its antioxidant property as a result of the presence of adjacent hydroxyl (OH) groups on the same ring (Cuevas et al., 2009; Haque et al., 2006; Mohamed et al., 2011; Rahman, 2016). In the present study, the chemical analysis of green tea extract indicated the presence of many free radical scavenging constituents including gallic acid, (-)-gallocatechin, (-)-gallocatechin, (-)-epicatechin, (-)-epigallocatechin, (-)-epigallocatechin gallate, (-)-gallocatechin gallate, (-)-epicatechin gallate, (-)-catechin gallate, and caffeine.
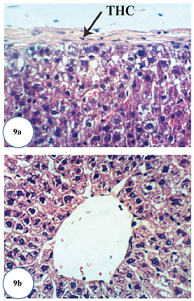 | Figure 9. Photomicrographs of liver sections of Dox-administered rats treated with epicatechin. (a) Slight thickening of hepatic capsule (THC) (H&E ×400) and (b) apparent normal hepatocytes (H&E ×400).
[Click here to view] |
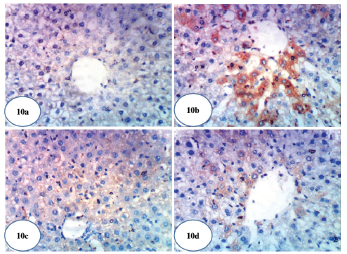 | Figure 10. Photomicrographs of immunohistochemical stained liver sections for COX-2 expression detection. (a) A photomicrograph of immunohistochemical staining of COX-2 in liver of normal control rat showing a weak expression of COX-2 (×400). (b) A photomicrograph of immunohistochemical staining of COX-2 in liver of Dox-administered rats showing a strong stimulated expression of COX-2 (immunopositivity indicated by brownish color) (×400). (c) A photomicrograph of immunohistochemical staining of COX-2 in liver of Dox-administered rats treated with green tea showing a weak expression of COX-2 (immunopositivity indicated by brownish color) (×400). (d) A photomicrograph of immunohistochemical staining of COX-2 in liver of Dox-administered rats treated with epicatechin showing a weak expression of COX-2 (immunopositivity indicated by brown color) (×400).
[Click here to view] |
Tumor marker AFP, a specific glycoprotein secreted from fetal liver and yolk sac, that rapidly falls few weeks after birth (Patil et al., 2013), is the most reliable serum marker for the diagnosis of hepatocellular carcinoma (HCC) (Wen-Jun et al., 2013). There was a marked elevation in serum AFP level in rats injected with Dox alone. This finding is consistent with the previous report which revealed that the injection of Dox to rats resulted in the increase of serum AFP level reflecting increased probability of and susceptibility to HCC (Kim et al., 2010). On the other hand, the Dox-injected groups treated with green tea and epicatechin showed an improvement in the serum level of AFP reflecting their potent anti-cancer and hepatoprotective effects. In agreement with the previous publication of Abozalam et al. (2016), Nakagami (2004), Rani et al. (2014), Shanmugam et al. (2017), and Tadesse et al. (2015) who reported the anticancer and antioxidant effects of green tea and epicatechin.
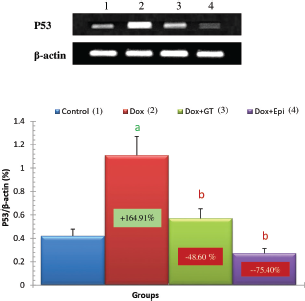
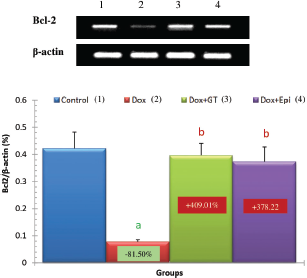
In the present study, the apoptotic mediators, p53, and caspase 3 expressions in liver were markedly elevated in Dox-administered rats while expression of anti-apoptotic marker Bcl-2 was significantly lowered. The treatment of Dox-administered rats with green tea aqueous extract and epicatechin prevented these alterations. The augmented apoptosis in Dox-administered rats may be attributed to the activated oxidative stress. This attribution was supported by Patel et al. (2010) who stated that oxidative stress has been implicated as a contributor factor to various forms of cell death, involving a specific inducer of apoptosis. In turn, the reduced apoptosis in the liver as a result of the treatment of Dox-injected rats with green tea aqueous extract and epicatechin may result secondary to the suppressed oxidative stress and enhanced antioxidant defense system. This suggestion runs parallel with Spencer et al. (2001) who evidenced that 3′-O-methyl epicatechin inhibits H2O2-induced cell death and that the mechanism involves attenuation of caspase-3 activity as an apoptotic marker.
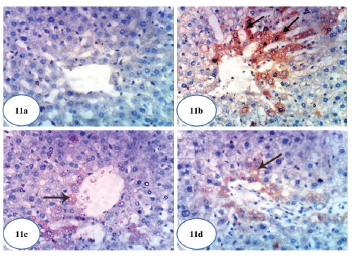 | Figure 11. Photomicrographs of immunohistochemical stained liver sections for caspase-3 expression detection. (a) A photomicrograph of immunohistochemical staining of caspase-3 in liver of normal control rat showing a weak expression of caspase-3 (×400). (b) A photomicrograph of immunohistochemical staining of caspase-3 in liver of Dox-administered rats showing a strong augmented expression of caspase-3 (immunopositivity indicated by brownish color) (×400). (c) A photomicrograph of immunohistochemical staining of caspase-3 in liver of Dox-administered rats treated with green tea showing a weak expression of caspase-3 (immunopositivity indicated by brownish color) (×400). (d) A photomicrograph of immunohistochemical staining of caspase-3 in liver of Dox-administered rats treated with epicatechin showing a weak expression of caspase-3 (immunopositivity indicated by brownish color) (×400).
[Click here to view] |
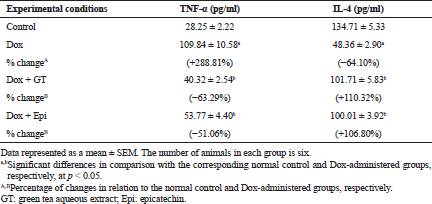
To assess the effect on the inflammatory status, the levels of serum TNF-α and IL-4 were determined by ELISA, liver NF-κB mRNA expression was assayed by RT-PCR, and COX-2 was assayed by immunohistochemical technique.
In the current study, the level of pro-inflammatory cytokine TNF-α in serum was significantly elevated in Dox-administered rats while the level of anti-inflammatory cytokine IL-4 was significantly decreased reflecting the preponderance of T helper 1 (Th1) and the presence of elevated Th1: T helper 2 (Th2) cell ratio. These data are in concurrence with Shankar et al. (2007) who found that injection of Dox augments a peripheral increase in the cytokine TNF-α, which stimulates several inflammatory pathways. As indicated by Tangpong et al. (2006), TNF-α induces mitochondrial malfunction by its downstream consequences, leading to further increase in cytochrome C release, oxidative stress, caspase 3 activity, and TUNEL-positive cell death, all of which are implicated as inducers of apoptosis following Dox injection. On the other hand, IL-4 was reported to inhibit multiple functions of activated macrophages, including macrophage production of TNF-α and interleukin-1β (IL-1β), and the macrophage secretion of reactive oxygen intermediates and up-regulate expression of interleukin-1 (IL-1) receptor antagonist (Abramson and Gallin, 1990; Hart et al., 1989; Vannier et al., 1992). It also activates macrophage 15 lipoxygenase activity, which may suppress the synthesis of pro-inflammatory leukotriene B4 (LTB4) (Katoh et al., 1994). The treatments of Dox-administered rats with green tea aqueous extract and epicatechin, in the present study, improved the deteriorations in both TNF-α and IL-4 levels reflecting dominance of Th2 cells. Thus, both green tea aqueous extract and epicatechin may have potent anti-inflammatory effects by activating the production Th2 cytokines and suppressing the activity of Th1 cells.
It was also evidenced in the present study that the liver NF-κB and COX-2 expressions were remarkably increased in Dox-administered rats and were recovered toward normal levels as a result of treatments of Dox-administered rats with green tea aqueous extract and epicatechin. These results are in concurrence with many previous reports. Lagha and Grenier (2015) found that black and green tea polyphenols suppress the inflammatory response of monocytes/macrophages mediated by Fusobacterium nucleatum (F. nucleatum). They also first stated that the black and green tea extracts, theaflavins, (-)-epigallocatechin-3-gallate (EGCG) reduce the NF-κB activation induced by F. nucleatum in monocytes. It is also relevant here to mention that NF-κB is a key regulator of genes coding for inflammatory mediators including of interleukin-1β (IL-1β), TNF-α, IL-6, and C-X-C Motif Chemokine Ligand 8 (CXCL8) by macrophages (Lagha and Grenier, 2015). Many other publications revealed the inhibitory effects of EGCG, which is a major component of green tea on NF-κB activation as well as TNF-α and COX-2 expression (Aggarwal and Shishodia, 2006; Gupta et al., 2004; Shankar et al., 2008; Shimizu et al., 2004). In the same way, Mohamed et al. (2011) reported that the treatment with epicatechin prior to Dox in rats significantly prevented the increase in TNF-α, iNOS, and NF-κB expressions.
In conclusion, the C. sinensis aqueous extract and epicatechin have potent preventive effects against Dox-induced hepatotoxicity. The suppression of oxidative stress, the enhancement of antioxidant defense system, the modulatory effects of inflammatory signaling pathways, and anti-apoptotic actions all are implicated to prevent the Dox-induced hepatotoxicity and to improve the liver architecture and integrity. Thus, Camellia sinesis aqueous extract and epicatechin may be useful substances for patients treated with Dox.
REFERENCES
Abd El-Aziz MA, Othman AI, Amer M, El-Missiry MA. Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J Appl Toxicol, 2001; 21:469–73. CrossRef
Abozalam SB, Salama AA, Arbid MS, Ain Shoka AA, Abd El-Latif HA. Effect of barley, green tea and doxorubicin against N-dimethylnitrosamine induced hepatorenal toxicity in rats. Curr Sci Internat, 2016; 5:386–99.
Abramson SL, Gallin JI. IL-4 inhibits superoxide production by human mononuclear phagocytes. J Immunol, 1990; 144:625–30.
Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Bioch Pharmacol, 2006; 71:1397–421. CrossRef
Ahmed OM. Histopathological and biochemical evaluation of liver and kidney lesions in streptozotocin diabetic rats treated with glimepiride and various plant extracts. J Union Arab Biol, 2001; 16A:585–625.
Ahmed OM. Antihyperglycemic effects of water extract of Ulva lactuca and its polysaccharides in nicotinamide-streptozotocin-induced diabetic rats. Egypt J Zool, 2010; 54:273–97.
Ahmed OM, Abdul-Hamid MM, El-Bakry AM, Mohamed HM, Abdel Rahman FS. Effects of green tea infusion and epicatechin on doxorubicin-induced renocardiotoxicity in male albino rats. IJPSR, 2019; 10(5):1000–14.
Ahmed OM, Ashour MB, Fahim HI, AbouZid SF, Ahmed RG, Abdel Gaid MA. Punica granatum mitigates 7, 12-dimethylbenz [A] anthracene and CCl4-induced oxidative stress and hepatic precancerous lesions in Wistar rats. Indo Am J Pharm Res, 2016; 6(09):6493–510.
Ahmed OM, Hozayen WG, Abo Sree HT. Effects of ethanolic purslane shoot and seed extracts on doxorubicin-induced hepatotoxicity in albino rats. Life Sci J, 2013; 10(4):67–74.
Ahmed OM, Hassan MA, Abdel-Twab SM, Abdel Azeem MN. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed Pharmacother, 2017; 94:197–205. CrossRef
Ahmed RR, Abdella EM. Modulatory effects of rosemary leaves aqueous extract on doxorubicin-induced histological lesions, apoptosis and oxidative stress in mice. Iran J Cancer Prev, 2010; 3(1):1–22.
Alappat B, Sarna JA, Truong C. Anticancer and antioxidant properties of flavored green tea extracts. J Agric Life Sci, 2015; 2:15–24.
Al-Hilfy JHY. Effect of green tea aqueous extract on body weight, glucose level, and kidney functions in diabetic male albino rats. J Al-Nahrain Univ, 2012; 15(3):161–6. CrossRef
Ashok I, Sheeladevi R. Biochemical responses and mitochondrial mediated activation of apoptosis on long-term effect of aspartame in rat brain. Redox Biol, 2014; 2:820–31. CrossRef
Asiri YA. Probucol attenuates cyclophosphamide-induced oxidative apoptosis, p53 and Bax signal expression in rat cardiac tissues. Oxid Med Cell Longev, 2010; 3:308–16. CrossRef
Baba S, Osakabe N, Natsume M, Muto YT, Takizawa TJ, Terao J. In vivo comparison of the bioavailability of (+)-catechin, (-)-epicatechin and their mixture in orally administered rats. J Nutr, 2001; 131:2885–91. CrossRef
Bai P, Ye H, Xie M, Saxena P, Zulewski H, Hamri GC, Djonov V, Fussenegger M. A synthetic biology-based liver-protection device preventing acute liver injuries. J Hepatol, 2016; 65(1):84–94. CrossRef
Balachandar AV, Malarkodi KP, Varalakshmi P. Protective role of DL alpha-lipoic acid against adriamycin-induced cardiac lipid peroxidation. Hum Exp Toxicol, 2003; 22:249–54. CrossRef
Bancroft JD, Stevens A, Turner DR. Theory and practice of histological techniques. 4th edition, Churchil Living Stone, New York, London, San Francisco, Tokyo, p. 766, 1996.
Beutler E, Duren O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med, 1963; 61(5):882–8.
Canadian Council on Animal Care (CCAC). Guide to the care and use of experimental animals. CCAC, Ottawa, ON, Canada, pp. 1–298, 2010.
Chinta SJ, Kumar JM, Zhang H, Forman HJ, Andersen JK. Up-regulation of gamma-glutamyl transpeptidase activity following glutathione depletion has a compensatory rather than an inhibitory effect on mitochondrial complex I activity: implications for Parkinson's disease. Free Radic Biol Med, 2006; 40(9):1557–63. CrossRef
Chomzynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Annu Rev Biochem, 1987; 162:156–9. CrossRef
Cuevas E, Limón D, Pérez-Severiano F, Díaz A, Ortega L, Zenteno E, Guevara J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur J Pharmacol, 2009; 616(1–3):122–7. CrossRef
Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, Cozen W, Mack TM, Lu QY, Zhang ZF. Dietary flavonoid intake and lung cancer—a population-based case-control study. Cancer, 2008; 112(10); 2241-2248. CrossRef
Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med, 2009; 46:1589–97. CrossRef
Deepa PR, Varalakshmi P. Protective effect of low molecular weight heparin on oxidative injury and cellular abnormalities in adriamycin-induced cardiac and hepatic toxicity. Chemico Biol Interact, 2003; 146(2):201–10. CrossRef
Doumas BT, Watson WA, Biggs HG. Determination of serum albumin. J Clin Chem Acta, 1971; 31:87–9. CrossRef
EL-Hak HNG, Moustafa AA, Mansour SR. Influence of injection mode and antioxidant dietary on the toxicity of Doxorubicin as a chemotherapy drug. Gastroenterol Hepatol, 2018; 9(6):255–7. CrossRef
EL-Maraghy SA, Rizk SM, El-Sawalhi MM. Hepatoprotective potential of crocin and curcumin against iron overload induced biochemical alterations in rat. Afr J Biochem Res, 2009; 3(5):215–21.
Geoffrey KK, John KM, Naomi M, Simon KM. Qualitative phytochemical screening of Camellia sinensis and Psidium guajava leave extracts from Kericho and Baringo Counties. Int J Adv Biotechnol Res, 2014; 5(3):506–12.
Gendler S. ᵧGT. In: Kaplan A, Pesce AJ (eds.). Methods in clinical chemistry. The C.V. Mosby Co, St Louis, Toronto, Princeton, pp. 1120–3, 1984.
Giorno RA. Comparison of two immunoperoxidase staining methods based on the avidin–biotin interaction. Diagn Immunol, 1984; 2:161–6.
Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride induced liver toxicity in mice. J Pharmacol Pharmacother, 2012; 3(2):149–55.
Gupta S, Hastak K, Afaq F, Ahmad N, Mukhtar H. Essential role of caspases in epigallocatechin-3-gallatemediated inhibition of nuclear factor kappaB and induction of apoptosis. Oncogene, 2004; 23:2507–22. CrossRef
Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr, 2006; 136:1043–7. CrossRef
Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential anti-inflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci USA, 1989; 86:3803–7. CrossRef
Hussein AM, Ahmed OM. Regioselective one-pot synthesis and anti-proliferative and apoptotic effects of some novel tetrazolo[1,5-a] pyrimidine derivatives. Bioorg Med Chem, 2010; 18:2639–44. CrossRef
Ibrahim SS, Barakat MA, Helmy HS. Modulating effect of carvedilol on doxorubicin-induced cardiomyopathy and hepatic damage. J Am Sci, 2010; 6:20–32.
Injac R, Perse M, Obermajer N, Djordjevic-Milic V, Prijatelj M, Djordjevic A, Cerar A, Strukelj B. Potential hepatoprotective effects of fullerenol C60 (OH) 24 in Doxorubicin-induced hepatotoxicity in rats with mammary carcinomas. Biomaterials, 2008; 29(24–25):3451–60. CrossRef
Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative Stress by three distinct mechanisms. Free Radic Biol Med, 2001; 30:433–46. CrossRef
JabÅ‚oÅ„ska-Trypuć A, KrÄ™towski R, Kalinowska M, Åšwiderski G, Cechowska-Pasko M, Lewandowski W. Possible mechanisms of the prevention of doxorubicin toxicity by cichoric acid—antioxidant nutrient. Nutrients, 2018; 10(44):1–21. CrossRef
Jendrassik L, Grof P. Total and direct bilirubin. Biochem Z, 1938; 297:81.
Kaiserova H, Simunek T, Sterba M, den Hartog GJ, Schroterova L, Popelová O, Geršl V, KvasniÄková E, Bast A. New iron chelators in anthracycline-induced cardiotoxicity. Cardiovasc Toxicol, 2007; 7(2): 145-150. CrossRef
Kalender Y, Yel M, Kalender S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. The effects of vitamin E and catechin. Toxicology, 2005; 209(1):39–45. CrossRef
Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP. Recent advances on tea polyphenols. Front Biosci (Elite Ed), 2012; 4:111–31. CrossRef
Kar M, Mishra D. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol, 1976; 57(2):315–9. CrossRef
Karaman A, Fadillioglu E, Turkmen E, Tas E, Yilmaz Z. Protective effects of leflunomide against ischemia reperfusion injury of the rat liver. Pediatr Surg Int, 2006; 22:428–34. CrossRef
Kassner N, Huse K, Martin HJ, Gödtel-Armbrust U, Metzger A, Meineke I, Brockmöller J, Klein K, Zanger UM, Maser E, Wojnowski L. Carbonyl reductase 1 is a predominant Doxorubicin reductase in the human liver. Drug Metab Dispos, 2008; 36:2113–20. CrossRef
Katoh T, Lakkis FG, Makita N, Badr KF. Co-regulated expression of glomerular 12/15-lipoxygenase and interleukin-4 mRNAs in rat nephrotoxic nephritis. Kidney Int, 1994; 46:341–9. CrossRef
Khan N, Mukhtar H. Cancer and metastasis: prevention and treatment by green tea. Cancer Metastasis Rev, 2010; 29:435–45. CrossRef
Kim JD, Bae SH, Park JY, Han KH, Woo HY, Choi JY, Yoon SK, Jang BK, Hwang JS, Kim SG. A comparative study of high-dose hepatic arterial infusion chemotherapy and trans-arterial chemoembolization using doxorubicin for intractable, advanced hepatocellular carcinoma. Korean J Hepatol, 2010; 16(4):355–61. CrossRef
King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist, 2001; 6:162–76. CrossRef
Kostyuk VA, Potapovich AI, Strigunova EN, Kostyuk TV, Afanas’ev IB. Experimental evidence that flavonoid metal complexes may act as mimics of superoxide dismutase. Arch Biochem Biophys, 2004; 428:204–8. CrossRef
Kuznetsova AV, Raimund M, Albert A, Valdur S, Michael G. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in Doxorubicin -induced cell death. Biochim Biophys Acta, 2011; 1813(6):1144–52. CrossRef
Lagha AB, Grenier D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci Rep, 2015; 6(34520):1–11. CrossRef
Lecumberri E, Dupertuis YM, Miralbell R, Pichard C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin Nutr, 2013; 32(6):894–903. CrossRef
Mandziuk S, Gieroba R, Korga A, Matysiak W, Jodlowska-Jedrych B, Burdan F, Poleszak E, Kowalczyk M, Grzycka-Kowalczyk L, Korobowicz E, Jozefczyk A. The differential effects of green tea on dose-dependent doxorubicin toxicity. Food Nutr Res, 2015; 59:29754. CrossRef
Mannervik B, Guthenberg C. Glutathione transferase (human placenta). In: Jakoby WB (ed.) Methods in enzymology. Academic Press, New York, NY, vol. 77, pp. 231–5, 1981. CrossRef
Marklund S, Marklin G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem, 1974; 47:469–74. CrossRef
Mohamed RH, Karam RA, Amer MG. Epicatechin attenuates Doxorubicin-induced brain toxicity: critical role of TNF-α, iNOS and NF-κB. Brain Res Bull, 2011; 86(1–2):22–8. CrossRef
Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr, 2000; 71(6 Suppl):1698S–702S. CrossRef
Murray R. Alanine aminotransferase. In: Kaplan A, Pesce AJ (eds.). Methods in clinical chemistry. The C.V. Mosby Co, St Louis, Toronto, Princeton, pp. 1088–90, 1984a.
Murray R. Aspartate aminotransferase. In: Kaplan A, Pesce AJ (eds.). Methods in clinical chemistry. The C.V. Mosby Co, St Louis, Toronto, Princeton, pp. 1112–6, 1984b.
Nakagami T; DECODA Study Group. Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia, 2004; 47:385–94. CrossRef
Okamoto M, Leung KP, Ansai T, Sugimoto A, Maeda N. Inhibitory effects of green tea catechins on protein tyrosine phosphatase in Prevotella intermedia. Oral Microbiol Immunol, 2003; 18(3):192–5. CrossRef
Panjrath GS, Patel V, Valdiviezo CI, Narula N, Narula J, Jain D. Potentiation of doxorubicin cardiotoxicity by iron loading in a rodent model. J Am Coll Cardiol, 2007; 49:2457–64. CrossRef
Patel N, Joseph C, Corcoran GB, Ray SD. Silymarin modulates Doxorubicin-induced oxidative stress, Bcl-xL and p53expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol, 2010; 245(2):143–52. CrossRef
Patil M, Sheth KA, Adarsh CK. Elevated alpha fetoprotein, no hepatocellular carcinoma. J Clin Exp Hepatol, 2013; 3:162–4. CrossRef
Pedrycz A, Czerny K. Immunohistochemical study of proteins linked to apoptosis in rat fetal kidney cells following prepregnancyadriamycin administration in the mother. Acta Histochem, 2008; 110:519–23. CrossRef
Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res, 2006; 124:491–504.
Preuss HG, Jarrel ST, Scheckenobac R, Lieberman S, Anderson RA. Comparative effects of chromium vanadium of Gymnema sylvester on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr, 1998; 17(2):116–23. CrossRef
Quine SD, Raghu PS. Effects of (-)-epicatechin, a flavonoid on lipid peroxidation and antioxidants in streptozotocin-induced diabetic liver, kidney and heart. Pharmacol Rep, 2005; 57:610–5.
Rahman I. Comparative analysis of phytochemical constituents, antibacterial and antioxidant activity of green tea (Camellia sinensis). A Dissertation submitted to Brac University in partial fulfilment of the requirements for the Bachelor of Science in Biotechnology, Biotechnology Programme, Department of Mathematics and Natural Sciences BRAC University Dhaka, Bangladesh, pp. 1–74, 2016.
Rahmani AH, Al shabrimi FM, Allemailem KS, Aly SM, Khan MA. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signaling pathway. BioMed Res Int, 2015; Article ID 925640:1–2. CrossRef
Rani R, Nagpal D, Gullaiya S, Madan S, Agrawal SS. Phytochemical, pharmacological and beneficial effects of green tea. Int J Pharmacogn Phytochem Res, 2014; 6(3):420–6.
Rizvi SI, Zaid MA. Intracellular reduced glutathione content in normal and type 2 diabetic erythrocytes: effect of insulin and (-)-epicatechin. J Physiol Pharmacol, 2001; 52:483–8.
Rizvi SI, Zaid MA, Anis R, Mishra N. Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin Exp Pharmacol Physiol, 2005; 32:70–5. CrossRef
Rozza AL, Hiruma-Lima CA, Tanimoto A, Pellizzon CH. Morphologic and pharmacological investigations in the epicatechin gastroprotective effect. Evid Based Complement Alternat Med, 2012; Article ID 708156:1–8. CrossRef
Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute Doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharma Col Res, 2001; 43:211–8. CrossRef
Sayed-Ahmed MM, Al-Shabanah OA, Hafez MM, Aleisa AM, Al-Rejaie SS. Inhibition of gene expression of heart fatty acid binding protein and organic cation/carnitine transporter in doxorubicin cardiomyopathic rat model. Eur J Pharmacol, 2010; 640:143–9. CrossRef
Sendensky A, Dufour JF. Liver physiology. In: Ginès P, Kamath P, Arroyo V (eds.). Chronic liver failure (mechanisms and management), clinical gastroenterology. Humana Press, Springer Science & Business Media, LLC, Springer Nature, Switzerland, pp. 33–45, 2011. Available via https//www.springer.com/gp/book/9781607618652. doi:10.1007/978-1-60761-866-9_2 CrossRef
Shankar S, Chen Q, Srivastava RK. Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to enhance antiangiogenic effects of EGCG through activation of FOXO transcription factor. J Mol Signal, 2008; 3(7):1–11. CrossRef
Shankar S, Suthakar G, Srivastava RK. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front Biosci, 2007; 12:5039–51. CrossRef
Shanmugam B, Shanmugam KR, Ravi S, Subbaiah GV, Ramakrishana C, Mallikarjuna K, Reddy KS. Exploratory studies of (-)-epicatechin, a bioactive compound of Phyllanthus niruri, on the antioxidant enzymes and oxidative stress markers in D-galactosamine-induced hepatitis in rats: a study with reference to clinical prospective. Pharmacogn Mag, 2017; 13:S56–62. CrossRef
Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res, 2005; 11:2735–46. CrossRef
Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood and the brain. Phytomed, 2000; 9:232–8. CrossRef
Spencer JP, Schroeter H, Kuhnle G, Srai SK, Tyrrell RM, Hahn U, Rice-Evans C. Epicatechin and its in vivo metabolite, 3'-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3activation. Biochem J, 2001; 354(Pt 3):493–500. CrossRef
Subhashini R, Mahadeva Rao US, Sumathi P, Gunalan G. A comparative phytochemical analysis of cocoa and green tea. Indian J Sci Technol, 2010; 3(2):188–92.
Swanston-Flatt SK, Day C, Flatt PR, Bailey CJ. Evaluation of antihyperglycemic properties of traditional plant treatment for diabetes in streptozotocin diabetic and db/db mice. In: Shafrir E (ed.). Frontiers in diabetes research. Lesson for animal diabetes. Smith-Gordon, London, UK, vol. 3, pp. 286–93, 1990.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol, 2013; 65(2):157–70. CrossRef
Tadesse A, Hymete A, Bekhit AA, Mohammed SF. Quantification of total polyphenols, catechin, caffeine, l-theanine, determination of antioxidant activity and effect on antileishmanial drugs of Ethiopian tea leaves extracts. Phcog Res, 2015; 7:7–14. CrossRef
Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, Clair WS, Ratanachaiyavong S, Clair DK, Butterfield DA. Butterfield, adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis, 2006; 23:127–39. CrossRef
Therland KL, Stubbe J, Thiesson HC, Ottosen PD, Walter S, Sorensen GL, Skøtt O, Jensen BL. Cycloxygenase-2 is expressed in vasculature of normal and ischemic adult human kidney and is colocalized with vascular prostaglandin E2 EP4 receptors. J Am Soc Nephrol, 2004; 15:1189–98. CrossRef
Tietz NW, Rinker AD, Shaw LM. International Federation of Clinical Chemistry. IFCC methods for the measurement of catalytic concentration of enzymes. Part 5. IFCC method for alkaline phosphatase (orthophosphoric-monoester phosphohydrolase, alkaline optimum, EC 3.1.3.1). IFCC Document Stage 2, Draft 1, 1983-03 with a view to an IFCC Recommendation. Clin Chim Acta, 1983; 135(3):339F–67F.
Trivedi PP, Kushwaha S, Tripathi DN, Jena GB. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Food Chem Toxicol, 2011; 11(3):215–25. CrossRef
Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin 4: Interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Nat Acad Sci USA, 1992; 89:4076–80. CrossRef
Vasconcelos PC, Seito LN, Di Stasi LC, Akiko Hiruma-Lima C, Pellizzon CH. Epicatechin used in the treatment of intestinal inflammatory disease: an analysis by experimental models. Evid Based Complement Alternat Med, 2012; article ID 508902:1–12. CrossRef
Wang G, Zhang J, Liu L, Sharma S, Dong Q. Quercetin potentiates Doxorubicin mediated antitumor effects against liver cancer throughp53/Bcl-xl. PLoS One, 2012; 7:51764. CrossRef
Wen-Jun M, Hai-Yong W, Li-Song T. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol, 2013; 11:212–9. CrossRef
Xi L, Zhu S, Das A, Chen D, Durrant, Hobbs DC, Lesnefsky EJ, Kukreja RC. Dietary inorganic nitrate alleviates doxorubicin cardiotoxicity: mechanisms and implications. Nitric Oxide, 2012; 26(4):274–84. CrossRef
Xin Y, Li-Li W, Wan J, Peng X, Cheng G. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem Toxicol, 2011; 49(1):259–64. CrossRef
Yagmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, Songur A. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res, 2007; 38(4):380–5. CrossRef
Yang XL, Fan CH, Zhu HS. Photo-induced cytotoxicity of malonic acid [C60] fullerene derivatives and its mechanism. Toxicol In Vitro, 2002; 16:41–6. CrossRef
Yanishlieva-Maslarowa NN, Heinonen IM. Sources of natural antioxidants: vegetables, fruits, herbs, spices and teas. In: Pokorny J, Yanishlieva N, Gordon M (eds.). Antioxidants in food– practical applications. CRC Press, Woodhead Publ., Cambridge, UK, pp. 210–49, 2001. CrossRef
Yoshida T, Majors RE. High-speed analyses using rapid resolution liquid chromatography on 1.8-µ porous particles. J Sep Sci, 2006; 29:2421–32. CrossRef
Zhao X, Zhang J, Tong N, Chen Y, Luo Y. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol Pharm Bull, 2012; 35(5):796–800. CrossRef

.png)