INTRODUCTION
Constructing novel pharmacologically interesting hybrid compounds for drug discovery was an important strategy in designing novel active leads (D’hooghe et al., 2012; Walsh and Bell, 2009). Indole derivatives represent one of the most important nitrogen heterocycles owing to their interesting widespread biological activities. Indole compounds with aryl substituted moieties have been characterized with different biological activities. The acrylamide derivatives of 2-phenylndole system found applications as estrogenic agents (Miller et al., 1999). Good binding behavior to DNA double helix was achieved by the 4’,6-dicarboxyamide derivative of 2-phenylindole hydrochloride salt as well as its diamidine analog (Kapuscinski and Skoczylas, 1978). Furthermore, a number of 2-phenylindole derivatives are also utilized as anti-sunburn agents (Jacquet et al., 1985). A number of 2-phenyl-3-sulfonylphenylindoles isomers and their derivatives have been revealed to possess selectively a potent cyclo-oxygenase enzyme (COX-2) inhibition activity (Hu et al., 2003). N-Substituted indoles I and II have significant GABAA agonist (Falcó et al., 2006) and good GPRC6A antagonist (Johansson et al., 2013) activity, respectively while compound III (Figure 1) has the ability of binding to the FtsZ domain of ZipA protein which can be possible antibacterial candidate via inhibition of cell division (Jennings et al., 2004). Moreover, the cytotoxicity and topoisomerase I/II inhibition effect of 2-phenylindole glycosides was studied (Shi et al., 2011). Indole linked heterocyclic hybrids having pyrazolyl-oxadaizole glycoside IV has promising anticancer activity (El-Sayed et al., 2017).
Imidazole derivatives have gained enormous attention because of their considerable biological activities (Vik et al., 2007; Li et al., 2006; Miyachi et al., 1999) including antitumor activity (Fortuna et al., 2008; Ballistreri et al., 2004; Cui et al., 2003). 2-Substituted indolyl-imidazole derivatives were synthesized and were revealed to possess CYP19 inhibition effect (Wang et al., 2013). As a compound incorporating both imidazole and indole motifs, Trachycladindole V (Capon et al., 2008) is an indolyl-imidazole derivative exhibiting potent cytotoxic activities against leukemia cells and lung cancer cells.
On the other hand, thiazolo-s-triazines were reported with their antifolate activity (Dolzhenko and Dolzhenko, 2006; Toyoda et al., 1997) and were progressed also as anticancer, antiparasitic, antibacterial, and antifungal agents (Chan and Anderson, 2006; Kompis et al., 2005; Gangjee et al., 2007). We have been interested in the synthesis of novel indole, thiazole and imidazole compounds and their sugar derivatives (El-Sayed et al., 2016; Flefel et al., 2017; Abdel-Rahman et al., 2012; Ali et al., 2012) searching for novel biologically active leads. To validate the effective integration of the anticancer activities of substituted indole and the potent cytotoxic activities of imidazole, triazine, and thiazole derivatives, our attention was directed to the synthesis of the hybridizing compounds of indole linked to imidazole or triazine moieties, and their fused analogs with thiazole system.
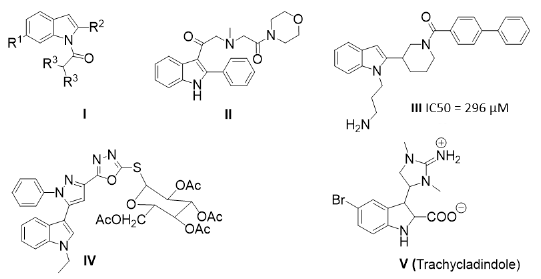 | Fig. 1: Active 2-substituted indoel and indolvl-imidazole derivatives. [Click here to view] |
MATERIALS AND METHODS
Instruments and reagents
All melting points are uncorrected and measured utilizing Electro-thermal IA 9100 appliance (Shimadzu, Tokyo, Japan). IR spectra have been enrolled as KBr pellets on a Perkin-Elmer 1650 spectrophotometer (Perkin-Elmer, Norwalk, CT, USA). 1H NMR has been attained on a Jeol-Ex-400 NMR spectrometer (Jeol, Tokyo, Japan) and chemical shifts were written as part per million; ppm (δ values) against TMS as interior standard. Mass spectra were investigated on VG 2AM-3F mass spectrometer (Thermo electron corporation, USA). Microanalyses were operated using Mario El Mentar apparatus and satisfactory results were within the accepted range (±0.30) of the calculated values. The reactions have been pursuit and the purity of synthesized compounds was checked by means of TLC on silica gel-protected aluminum sheets (Type 60 F254, Merck). All used chemicals were of reagent grade and were used as supplied directly unless otherwise stated.
2-Chloro-1-(2-phenyl-1H-indol-1-yl)ethanone (2)
A fully stirred mixture of 2-phenyl-1H-indole (1) (1.93 g, 0.01 mol), chloroacetylchloride (1.13g, 0.01 mol) and pyridine (1 mL) in toluene (50 mL) was heated under reflux temperature for 8 h. Toluene was evaporated from the reaction mixture unto dryness. The appeared precipitate was collected by filtration and re-crystallized from ethanol and compound 2 was resulted as a brownish powder, Yield 81%, m.p.165-168°C. IR (KBr, cm-1) ν: 3100 (CH aromatic), 2910 (CH aliphatic) and 1692 (C=O), 1H NMR (DMSO-d6) δ ppm: 4.80 (s, 2H, CH2), 6.90 (s, 1H, Ar-H), 7.20-7.26 (m, 2H, Ar-H), 7.23-7.29 (m, 2H, Ar-H), 7.40-7.47 (m, 3H, Ar-H), 7.85-7.91 (m, 2H, Ar-H). MS m/z: 269 (100%, M+), 271.70 (31%, M+2). Anal. calcd. For C16H12ClNO (269.7): C, 71.25; H, 4.48; N, 5.19. Found: C, 71.60; H, 4.57, N, 5.15.
General procedure for the preparation of N-(imidazolyl)indole derivatives 3a,b
To a warmed ethanolic potassium hydroxide solution [potassium hydroxide (0.56 g, 0.01 mole) in ethanol (50 mL)] a mixture of the indole derivative 2 (2.69 g, 0.01 mole) and urea or thiourea (0.01 mole) has been provided. The reaction flask was heated under reflux for two h, then cooled and poured onto ice-water. The solid product was filtered off and recrystallized from ethanol to afford derivatives 3a,b respectively.
4-(2-Phenyl-1H-indol-1-yl)-1,5-dihydro-2H-imidazol-2-one (3a)
Brownish powder; Yield: 64%; m.p. 130-132°C. IR (KBr, cm-1) ν: 3320 (NH), 3090 (CH aromatic), 2940 (CH aliphatic), 1645 (C=O), 1186 (C=S). 1H NMR (DMSO-d6) δ ppm: 3.42 (s, 2H, CH2), 6.30 (s, 1H, Ar-H), 7.20 (d, 1H, J = 6.2 Hz, Ar-H), 7.40-7.50 (m, 3H, Ar-H), 7.60-7.69 (m, 3H, Ar-H), 8.09 (m, 2H, Ar-H), 12.50 (brs, 1H, NH). 13C NMR (DMSO-d6) δ ppm: 81.0 (CH2), 102.0, 112.0, 119.5, 120.0, 121.0, 122.5, 127.0, 128.5, 129.0, 129.2, 130.3, 132.2 (Ar-C), 168.0 (C=N), 175.0 (C=O). MS (m/z)%: 275.3 (M+, 51%). Anal. calcd. For C17H13N3O (275.3): C, 74.17; H, 4.76; N, 15.26. Found: C, 74.21; H, 4.82; N, 15.23.
4-(2-Phenyl-1H-indol-1-yl)-1,5-dihydro-2H-imidazole-2-thione (3b)
Brownish powder; Yield: 73%; m.p. 250-252°C. IR (KBr, cm-1) ν: 3310 (NH), 3150 (CH aromatic) and 2950 (CH aliphatic).1H NMR (DMSO-d6) δ ppm: 3.24 (s, 2H, CH2), 7.20 (d, 1H, J = 6.2 Hz, Ar-H), 7.40 (s, 1H, Ar-H), 7.43-7.52 (m, 3H, Ar-H), 7.55-7.62 (m, 3H, Ar-H), 7.99-8.05 (m, 2H, Ar-H), 12.19 (brs, 1H, NH). 13C NMR (DMSO-d6) δ ppm: 66.0 (CH2N), 112.0, 122.0, 122.3, 122.5, 122.8, 122.9, 130.0, 126.0, 126.3, 128.0, 128.2, 128.3 (12 Ar-C), 167.0 (C=N), 194.0 (C=S). MS (m/z)%: 291.08 (M+, 41%). Anal. calcd. For C17H13N3S: 291.37: C; 70.08; H, 4.50; N, 14.42. Found: C, 70.18; H, 4.61; N, 14.39.
5-(2-Phenyl-1H-indol-1-yl)-1,2-dihydro-1,2,4-triazine-3(6H)-thione (4)
The N-substituted indole derivative 2 (2.69 g, 0.01 mole) and thiosemicarbazide (0.91 g, 0.01 mole) were added sequentially to a warmed ethanolic sodium hydroxide solution [KOH (0.40 g, 0.01 mole) in ethanol (50 mL)]. The obtained mixture was refluxed for 15 minutes, then cooled and poured onto crushed ice. The formed solid product was filtered-off and recrystallized from ethanol to form the triazine derivative 4 as a brownish powder, Yield 74%; m.p. 180-182°C. IR (KBr, cm-1) ν: 3350 (NH), 3050 (CH aromatic) and 2980 (CH aliphatic); 1H NMR (DMSO-d6) δ ppm: 3.40 (s, 2H, CH2N-), 6.30 (s, 1H, Ar-H), 7.23-7.33 (m, 4H, Ar-H), 7.54-7.62 (m, 3H, Ar-H), 7.95-8.02 (m, 2H, Ar-H), 8.99-9.05 (brs, 2H, 2NH). 13C NMR (DMSO-d6) δ ppm: 50.0 (CH2NH), 135.6 (triazine C-5), 108.5, 110.7, 122.9, 129.2, 130.5, 130.9, 131.8, 133.8, 134.0, 134.7, 136.0, 136.8 (Ar-C), 179.3 (C=S). MS, m/z (%): 306.3 (M+, 41%). Anal. calcd. For C17H14N4S (306.38): C, 66.64; H, 4.61; N, 18.29; S, 10.47. Found: C, 66.72; H, 4.70; N, 18.25; S, 10.43.
6-(2-Phenyl-1H-indol-1-yl)-5,7a-dihydroimidazo[2,1-b]thiazol-3(2H)-one (5)
Compound 3b (2.91 g, 0.01 mole), chloroacetic acid (0.95 g, 0.01 mole) and anhydrous sodium acetate (0.02 mole) were added to a flask containing glacial acetic acid (30 mL)/acetic anhydride (15 mL) mixture followed by heating at reflux temperature for three h. The cooled reaction mixture and treatment with ice-cold water resulted in the formation of a precipitate which was filtered off, dried and recrystallized from ethanol to give compound 5.
Dark brownish powder; Yield: 71%; m.p. 203-205°C. IR (KBr, cm-1) ν: 3090 (CH aromatic), 2965 (CH aliphatic) and 1665 (C=O). 1H NMR (DMSO-d6) δ ppm: 3.20 (s, 2H, CH2S), 3.40 (s, 2H, CH2N), 4.20 (s, 1H, thiazole H-2), 6.95 (s, 1H, Ar-H), 7.30-7.36-742 (m, 2H, Ar-H), 7.40-7.52 (m, 5H, Ar-H), 8.20-8.25 (m, 2H, Ar-H). 13C NMR (DMSO-d6) δ ppm: 39.3 (CH2S), 39.5 (CH2N), 41.9 (thiazole C-2), 128.1, 128.5, 128.7, 128.8, 128.9, 128.92, 128.98, 129.1, 129.3, 129.8, 130.2, 130.3 (Ar-C), 159.4 (C=N), 165.5 (C=O). MS, m/z (%): 333 (M+, 49%). Anal. calcd. For C19H15N3OS (333.41): C, 68.45; H, 4.53; N, 12.60. Found: C, 68.29; H, 4.59; N, 12.51.
2-(4-Chlorobenzylidene)-6-(2-phenyl-1H-indol-1-yl)-5,7a-dihydroimidazo[2,1-b]thiazol-3(2H)-one (6)
A mixture of compound 3b (2.91 g, 0.01 mole), chloroacetic acid (0.95 g, 0.01 mole), p-chlorobenzaldehyde (1.40 g, 0.01 mole) and anhydrous sodium acetate (0.02 mole) was refluxed for three hours in (30 mL) of glacial acetic acid containing acetic anhydride (15 mL). The reaction mixture was cooled and poured onto ice-cold water. The afforded precipitated solid was filtered off, dried and recrystallized from ethanol to produce compound 6.
Brownish powder; Yield: 74%, m.p. 241-243°C. IR (KBr, cm-1) ν: 3140 (CH aromatic), 2900 (CH aliphatic), 1645 (C=O). 1H NMR (DMSO-d6) δ ppm: 3.45 (s, 2H, CH2N), 3.80 (s, 1H, thiazole H-2), 6.50 (s, 1H, Ar-H), 7.20 (m, 2H, Ar-H), 7.55-7.68 (m, 5H, Ar-H), 7.70-7.79 (m, 4H, Ar-H), 7.90-7.98 (m, 3H, Ar-H and CH=C). 13C NMR (DMSO-d6) δ ppm: 40.0 (CH2), 60.0 (thiazole C-2), 112.0 (CH=C), 122.0 (thiazole C-5), 124.0, 124.2, 128.0, 128.2, 128.3, 128.4, 130.0, 130.1, 130.2, 131.0, 134.0, 135.0 (Ar-C), 166.5 (C=N), 168.0 (C=O). MS, m/z (%): 455 (M+, 91%), 457 (M+2, 34%). Anal. calcd. For C26H18ClN3OS (455.96): C, 68.49; H, 3.98; N, 9.22. Found: C, 68.60; H, 3.88; N, 9.04.
7-(2-Phenyl-1H-indol-1-yl)-6,8a-dihydro-5H-thiazolo[3,2-b][1,2,4]triazin-3(2H)-one (7)
To a solution of compound 4 (3.06 g, 0.01 mole) in glacial acetic acid (30 mL) containing acetic anhydride (15 mL) was added chloroacetic acid (0.95 g, 0.01 mole) and anhydrous sodium acetate (0.02 mole) then the mixture was allowed to reach the reflux temperature and heating was continued for one hour. The precipitated black solid appeared after cooling and pouring into ice-cold water was collected via filtration, dried and re-crystallized from ethanol to produce 7. Dark brownish powder; Yield: 74%, m.p. 180-182°C. IR (KBr, cm-1) ν: 3360 (NH), 3100 (CH aromatic), 2985 (CH aliphatic), 1643 (C=O). 1H NMR (DMSO-d6) δ ppm: 3.30 (s, 2H, CH2S), 4.20 (s, 2H, CH2N), 4.50 (s, 1H, thiazole H-2), 6.50 (s, 1H, Ar-H), 7.30 (brs, 1H, NH), 7.40-7.53 (m, 4H, Ar-H), 7.90-8.05 (m, 5H, Ar-H). 13C NMR (DMSO-d6) δ ppm: 31.2 (CH2S), 41.6 (CH2N-), 60.9 (thiazole C-2), 138.9 (triazine C-5), 100.2, 110.5, 115.2, 120.2, 120.7, 124.1, 126.0, 127.1, 128.5, 130.8, 131.7, 133.6 (Ar-C), 169.3 (C=O). MS m/z (%): 348.10 (M+, 10%). Anal. calcd. For C19H16N4OS (348.4): C, 65.50; H, 4.63; N, 16.08. Found: C, 65.22; H, 4.58; N, 16.19.
2-(4-Chlorobenzylidene)-7-(2-phenyl-1H-indol-1-yl)-6,8a-dihydro-5H-thiazolo[3,2-b][1,2,4]triazin-3(2H)-one (8)
To the triazine derivative 4 (3.06 g, 0.01 mole) and chloroacetic acid (0.95 g, 0.01 mole) dissolved in glacial acetic acid (30 mL) containing acetic anhydride (15 mL), was added p-chlorobenzaldehyde (1.40 g, 0.01 mole) and anhydrous sodium acetate (0.02 mole) then the mixture was refluxed for four h. Cooling followed by addition of ice-cold water led to the formation of a precipitate which was filtered off, dried and recrystallized from ethanol to provide compound 8.
Brownish powder; Yield: 76%, m.p. 190-192°C. IR (KBr, cm-1) ν: 3380 (NH), 3150 (CH aromatic), 2975 (CH aliphatic) and 1665 (C=O). 1H NMR (DMSO-d6) δ ppm: 3.30 (s, 2H, CH2N), 3.90 (s, 1H, thiazole H-2), 7.20 (s, 1H, Ar-H), 7.40 (s, 1H, CH=C), 7.45 (brs, 1H, NH), 7.50-7.62 (m, 4H, Ar-H), 7.65-7.76 (m, 4H, Ar-H), 7.90-8.03 (m, 5H, Ar-H). 13C NMR (DMSO-d6) δ ppm: 46.2 (CH2N), 76.6 (thiazole C-2), 120.9, 131.9, 139.0, 110.1, 120.0, 122.1, 122.8, 125.1, 127.0, 128.0, 128.5, 130.0, 131.7, 133.6, 134.0, 136.1, 136.4, 136.8, 136.9 (Ar-C, thiazole C-5, triazine C-5 and CH=C), 167.3 (C=O). MS, m/z (%): 470 (M+, 73%), 472 (M+, 23%). Anal. calcd. For C26H19ClN4OS (470.97): C, 66.31; H, 4.07; N, 11.90. Found: C, 66.04; H, 4.02; N, 12.04.
2-(Acetoxymethyl)-6-(5-(2-phenyl-1H-indol-1-yl)-4H-imidazol-2-ylthio)tetrahydro-2H-pyran-3,4,5-triyl triacetate (9)
2,3,4,6-Tetra-O-acetyl-α-D-glucopyranosyl bromide (1.66 g, 0.005 mole) dissolved in acetone (15 mL) was added portion-wise to a clear solution of compound 3b (1.46 g, 0.005 mole) and potassium hydroxide (0.28 g, 0.005 mole) in distilled water (2 ml). The reaction mixture was stirred at room temperature until the reaction was judged complete by TLC (pet. ether/ethyl acetate, 4: 1 v/v). Evaporation of the solvent afforded a residue which was washed with distilled water (10 mL) followed by extraction with chloroform. The obtained residue after removal of chloroform was triturated with petroleum ether (b.p. 40-60°C) (45 mL) with stirring. The solid product was filtered, dried and recrystallized from ethanol to produce compound 9.
Brownish powder; Yield: 71%, m.p. 270-272°C. IR (KBr, cm-1) ν: 3100 (CH aromatic), 2985 (CH aliphatic) and 1745 (C=O). 1H NMR (DMSO-d6) δ ppm: 1.91, 193, 1.96, 2.01 (4s, 12H, 4CH3CO), 3.30 (s, 2H, CH2N), 4.05 (dd, 1H, J = 11.8, 3.4 Hz, H-6´), 4.10 (m, 1H, H-6´´), 4.48-4.51 (m, 1H, H-5´), 4.78-4.81 (m, 1H, H-4´), 5.17 (dd, 1H, J = 7.6, J = 9.8 Hz, H-2´), 5.37 (d, 1H, J = 9.8 Hz, H-1´), 5.58 (t, 1H, J = 7.6 Hz, H-3´), 7.30 (s, 1H, Ar-H), 7.80-7.92 (m, 4H, Ar-H), 8.38-8.47 (m, 5H, Ar-H). Anal. calcd. For C31H31N3O9S (621.66): C, 59.89; H, 5.03; N, 6.76. Found: C, 59.72; H, 4.91; N, 6.88.
Ethyl 2-(2-oxo-4-(2-phenyl-1H-indol-1-yl)-2,5-dihydro-1H-imidazol-1-yl)acetate (10)
Ethyl bromoacetate (1.67 g, 0.01 mole) was added to a mixture of sodium bicarbonate (0.84 g, 0.01mole) and compound 3a (2.75 g, 0.01 mole) in acetone (30 mL), then the reaction mixture was heated under reflux for 20 hours. The mixture was evaporated till dryness and washed with hot water. The formed precipitate was collected by filtration and recrystallized from ethanol to give the ester derivative10.
Brownish powder; Yield: 74%, m.p. 241-243°C. IR (KBr, cm-1) ν: 3135 (CH aromatic), 2980 (CH aliphatic), 1745 (C=O), 1655 (C=O). 1H NMR (DMSO-d6) δ ppm: 1.01 (t, 3H, J = 5.4 Hz, CH3), 3.30 (s, 2H, CH2N), 4.05 (q, 2H, J = 5.4 Hz, CH2), 4.85 (s, 2H, CH2), 6.30 (s, 1H, Ar-H), 7.26-7.37 (m, 4H, Ar-H), 7.60-7.68 (m, 3H, Ar-H), 8.19-8.24 (m, 2H, Ar-H). 13C NMR (DMSO-d6) δ ppm: 14.2 (CH3), 40.9, 51.9, 59.0 (3CH2), 110.1, 116.0, 120.2, 120.5, 120.9, 128.2, 128.5, 130.1, 130.8, 131.7, 134.0, 143.5 (Ar-C), 166.1 (C=N), 167.0, 167.2 (2 C=O). MS m/z (%): 361.0 (M+, 73%). Anal. calcd. For C21H19N3O3 (361.40): C, 69.79; H,5.30; N, 11.63. Found: C, 69.90; H, 5.44; N, 11.37.
2-(2-Oxo-4-(2-phenyl-1H-indol-1-yl)-2,5-dihydro-1H-imidazol-1-yl)acetohydrazide (11)
Hydrazine hydrate (99%) (9.5 mL) was added to a mixture of compound 10 (3.61 g, 0.01 mole) in ethanol (30 mL) and the mixture was heated under reflux for 2 hours. The resulting mixture was cooled and filtered-off then recrystallized from ethanol to produce 11.
Grey powder; Yield: 74%, m.p. 191-193° C. IR (KBr, cm-1) ν: 3450 (NH2), 3370 (NH), 3100 (CH aromatic), 2900 (CH aliphatic), 1680 (C=O), 1665 (C=O). 1H NMR (DMSO-d6) δ ppm: 3.70 (s, 2H, CH2), 4.02 (s, 2H, CH2), 4.88 (brs, 2H, NH2), 7.05 (s, 1H, Ar-H), 7.20-7.32 (m, 4H, Ar-H), 7.50-7.58 (m, 3H, Ar-H), 7.60-7.66 (m, 2H, Ar-H), 9.20 (brs, 1H, NH). 13C NMR (DMSO-d6) δ ppm: 41.2, 59.6 (2CH2), 103.5, 110.7, 120.2, 120.9, 128.2, 128.5, 128.9, 130.0, 130.8, 131.7, 133.0, 133.6 (Ar-C) 163.6 (CH=N), 167.3, 169.0 (2C=O). MS, m/z (%): 347.0 (M+, 61%). Anal. calcd. For C19H17N5O2 (347.37): C, 65.69; H, 4.93, N, 20.16. Found: C, 65.79; H, 4.98; N, 19.98.
N’-(4-Chlorobenzylidene)-2-(2-oxo-4-(2-phenyl-1H-indol-1-yl)-2,5-dihydro-1H-imidazol-1-yl)acetohydrazide (12)
A solution of compound 11 (3.47 g, 0.01 mole) and p-chlorobenzaldehyde (1.40 g, 0.01 mole) in ethanol (30 mL) containing a catalytic amount of piperidine was heated under reflux for four hours. The mixture was allowed to cool to room temperature and then it was poured into water, and then was allowed to stand in a refrigerator for 12 h. The formed precipitate was filtered off, washed with water, dried and recrystallized from ethanol to produce compound 12.
Brownish powder; Yield: 76%, m.p. 130-132°C. IR (KBr, cm-1) ν: 3380 (NH), 3050 (CH aromatic), 2910 (CH aliphatic), 1675 (C=O), 1635 (C=O), 1626 (C=N). 1H NMR (DMSO-d6) δ ppm: 3.30 (s, 2H, CH2), 4.00 (s, 2H, CH2), 6.30 (s, 1H, Ar-H), 6.80-6.87 (m, 2H, Ar-H), 7.03 (d, 2H, J = 8.2 Hz, Ar-H), 7.20-732 (m, 4H, Ar-H), 7.90-8.05 (m, 5H, Ar-H), 9.15 (brs, 1H, NH), 8.65 (s, 1H, CH=N). 13C NMR (DMSO-d6) δ ppm: 46.1, 57.6 (2CH2), 100.0, 115.6, 119.8, 120.7, 124.3, 128.2, 129.2, 130.0, 130.8, 131.7, 131.8, 132.1, 132.8, 133.0, 135.7, 136.6 (Ar-C), 144.2, 164.5 (CH=N), 168.1, 171.2 (2C=O). MS, m/z (%) 469 (M+, 90%), 471 (M+2, 34%). Anal. calcd. For C26H20ClN5O2 (469.9): C, 66.45; H, 4.29; N, 14.90. Found: C, 66.29; H, 4.12; N, 15.03.
General procedure for the preparation of sugar hydrazones 13 and 14
To a well-stirred solution of the hydrazide 11 in ethanol containing 3 drops of glacial acetic acid, D-galactose or D-ribose (0.015 mol) dissolved in distilled water (1 mL) was added. The reaction mixture was heated under reflux for 5 hours then the half of the solvent amount was evaporated under reduced pressure, then left to stand at room temperature overnight. The precipitated solid was filtered, washed with cold ethanol, dried and recrystallized from ethanol/DMF (3:1) to afford compounds 13 and 14, respectively.
2-(2-Oxo-4-(2-phenyl-1H-indol-1-yl)-2,5-dihydro-1H-imidazol-1-yl)-N’-(2,3,4,5,6-pentahydroxyhexylidene)acetohydrazide (13)
Brownish solid, Yield: 71%; m.p. 198-199°C. IR (KBr, cm-1) ν: 3490-3440 (OH), 3290 (NH), 3055 (CH aromatic), 2915 (CH aliphatic), 1668 (C=O), 1632 (C=N). 1H NMR (DMSO-d6) δ ppm: 3.38-3.46 (m, 4H, H-6´, 6´´ and CH2), 3.80-3.95 (m, 3H, H-5´ and CH2), 4.21 (m, 2H, H-3´, 4´), 4.48-4.61 (m, 2H, H-2´ and OH), 4.83-4.91 (m, 2H, 2OH), 4.97-5.12 (m, 1H, OH), 5.13 (m, 1H, OH), 6.17 (s, 1H, Ar-H), 6.44 (m, 1H, Ar-H), 7.18-7.25 (m, 3H, Ar-H), 7.28 (d, 1H, J = 7.5 Hz, H-1´), 7.51-70 (m, 3H, Ar-H), 7.73-7.77 (m, 1H, Ar-H), 8.19-8.22 (m, 1H, Ar-H), 9.40 (brs, 1H, NH). 13C NMR (DMSO-d6) δ ppm: 47.8, 59.6, 60.5 (2CH2), 64.1, 72.1, 74.0 (3CH), 105.0, 115.1, 119.2, 127.5, 128.2, 128.7, 129.4, 130.0, 130.8, 131.7, 133.1, 135.7 (Ar-C), 153.4, 164.0 (CH=N), 168.0, 171.0 (2C=O). Anal. calcd. For C25H27N5O7 (509.52): C, 58.93; H, 5.34; N, 13.75. Found: C, 58.69; H, 5.24; N, 13.93.
2-(2-Oxo-4-(2-phenyl-1H-indol-1-yl)-2,5-dihydro-1H-imidazol-1-yl)-N’-(2,3,4,5-tetrahydroxypentylidene)acetohydrazide (14)
Brownish solid, Yield: 69%; m.p. 196-197°C. IR (KBr, cm-1) ν: 3495-3455 (OH), 3280 (NH), 3055 (CH aromatic), 2927 (CH aliphatic), 1652 (C=O), 1666 (C=O), 1626 (C=N). 1H NMR (DMSO-d6) δ ppm: 3.39-3.49 (m, 4H, H-5´, 5´´and CH2), 3.55-3.57 (m, 1H, H-4´), 3.71-3.76 (m, 2H, CH2), 3.96-3.98 (m, 1H, H-3´), 4.18-4.21 (m, 2H, H-2´ and OH), 5.16-5.20 (m, 2H, 2OH), 5.39 (m, 1H, OH), 6.56 (s, 1H, Ar-H), 6.85-6.98 (m, 2H, Ar-H), 7.05-7.31 (m, 5H, Ar-H), 7.45 (d, 1H, J = 7.5 Hz, H-1´), 7.86-7.95 (m, 2H, Ar-H), 9.14 (brs, 1H, NH). 13C NMR (DMSO-d6) δ ppm: 47.8, 59.6, 60.5 (2CH2), 64.1, 72.1, 72.3, 74.0 (4CH), 105.0, 115.1, 119.2, 127.5, 128.2, 128.7, 129.4, 130.0, 130.8, 131.7, 133.1, 135.7 (Ar-C), 153.4, 164.0 (CH=N), 168.0, 171.0 (2C=O). Anal. calcd. For C24H25N5O6 (479.49): C, 60.12; H, 5.26; N, 14.61. Found: C, 59.91; H, 5.17; N, 14.83.
Cytotoxic Activity
Material
All cell lines were brought from ATCC via Vacsera tissue culture laboratories. All media were bought from Lonza, Belgium, serum from Gibco, trypsin, and MTT from Biobasic Canada.
In vitro antitumor bioassay on human tumor cell lines
Cell culture
The cell lines namely; MCF7, PC3 HepG2 and HCT116 were maintained in DMEM high glucose with l-glutamine, 10% fetal bovine serum at 37°C in 5% CO2 and 95% humidity. Cells were sub-cultured using trypsin-versene 0.15%.
Viability test
After about 24h of seeding 20000 cells per well (in 96-well plates), when cells have reached 60-70% confluence, the medium was converted to serum-free medium possessing a final concentration of the tested samples of 100 μM in triplicates. The cells were treated for 72 h. 100 μM of Doxorubicin has been used as a positive control and serum-free medium was used as a negative control.
IC50 calculation and statistical analysis—Exactly the same procedure in the viability test was done. Only in this case, the test samples which gave 90% cytotoxicity or more on the cells were chosen. Four concentrations of those test samples were tested (in triplicates) on the cell lines. The results obtained were analyzed statistically using the SPSS program performing non-linear regression analysis to obtain the IC50 values.
Cell viability was determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as reported (Mosmann, 1983)
The equation used for calculation of percentage cytotoxicity is as following:
where: Av: average, X: absorbance of the sample well measured at 595 nm with reference 690 nm, NC: absorbance of negative control measured at 595 nm with reference 690 nm.
RESULTS AND DISCUSSION
Chemistry
In the present work, the new heterocyclic hybrids of indole system were synthesized via attachment of heterocyclic motifs either mono- or bicyclic rings such as imidazole, triazine, thiazole or sugar moieties to 2-phenylindole. The nitrogen in 2-phenylindole 1 is a good nucleophile that is capable of attacking electrophilic species (Bandini, 2013). The starting compound 1 was reacted with chloroacetyl chloride to afford 2-(2-phenyl-1H-indol-1-yl)acetyl chloride 2 in 81% yield. The latter was allowed to react with urea, thiourea, and thiosemicarbazide to afford the 2-oxo- and 2-thioxo-substituted imidazolyl and triazinyl derivatives 3a,b and 4, respectively. The observed mode of heterocyclization which lead to the formation of compounds 3a,b and 4 is in accordance with the formation of cyclized product from similar related structures (Ramla et al., 2006). The infra-red spectra of compounds 2-4 showed the characteristic absorptions corresponding to the C=O and C=S group in addition to the NH bands. The methylene protons of 3a,b, and 4 compounds appeared in their corresponding 1H NMR spectra as singlet signal at δH3.24-3.42 ppm, in addition, the signals attributed to the other protons in their assigned structures. The 13C NMR spectrum of the imidazolyl-indoles 3a,b showed the characteristic signals assigned for the carbonyl and C=S groups, respectively. The indolyl linked imidazole-2-thione derivative 3b was reacted with, chloroacetic acid and the derived dihydroimidazo[2,1-b] thiazol-3(2H)-one derivative 5 was afforded. Furthermore, carrying out the reaction in existence of p-chlorobenzaldehyde gave the arylidine derivative of the bicyclic imidazo[2,1-b]thiazol-3(2H)-one 6. Similarly, the reaction of the triazinyl-indole derivative 4 with chloroacetic acid led to the formation of the fused bicyclic thiazolo[3,2-b][1,2,4]triazin-3(2H)-one derivative 7. Moreover, performing the reaction in presence of benzaldehyde resulted in the formation of the thiazolo[3,2-b][1,2,4]triazin-2(3H)-ylidene)methyl)benzaldehyde derivative 8 in 76% yield (Yousif et al., 2017). The IR spectra of compounds 5-8 revealed the presence of the characteristic bands in the C=O frequency in addition to NH bands in the triazine ring of compounds 7 and 8 (scheme 1). The 1H NMR of compound 5 and 7 showed the CH2signals which have been disappeared in the corresponding arylidine derivatives 6 and 8, respectively in addition to the appearance of signals assigned for the remaining protons the assigned structures.
On the other hand, glycosylation of the imidazole-2-thione by means of α-bromoacetoglucose produced the thioglycoside derivative 9. The thio-linkage mode of attachment of the heterocycl to the glycosyl moiety was confirmed by the afforded NMR data which showed the anomeric proton (H-1) at 5.37 ppm as a doublet with a coupling constant J = 9.8 Hz confirming the β-conformation (El-Sayed et al., 2008). In addition, the IR spectrum of the latter thioglycoside showed the bands corresponding to the acetyl-carbonyl groups.
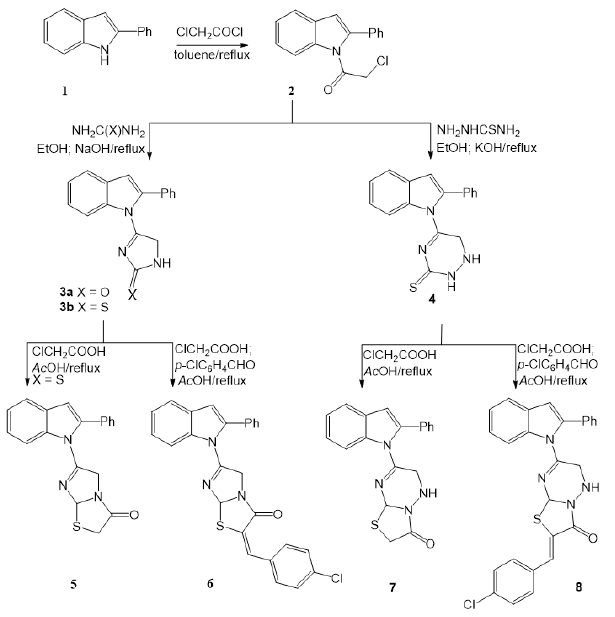 | Scheme 1: Synthesis of imidaolothiazole and triazine substituted indole derivatives. [Click here to view] |
The imidazolyl-indole derivative 3a was also reacted with ethyl bromoacetate in alkaline medium and gave the derived ethyl ester compound 10 in 74% yield. The reaction of the latter acetate derivative with hydrazine hydrate afforded the corresponding ((indolyl)imidazol-1-yl)acetohydrazide derivative 11. When the acid hydrazide 11 was allowed to react with p-chlorobenzaldehyde, the corresponding aryl hydrazone compound 12 was formed. The 1H NMR of the ester 10 indicated the presence of the ethyl group which have been shown by its characteristic signals as triplet and quartet at δH 1.00 and 4.85, respectively which in turn disappeared in its hydrazide derivative 11. Characteristic signal in the aromatic region were shown in the 1H NMR of the aryl hydrazone 12 in addition to the methane proton at δH8.65 ppm. The 13C NMR of compounds 10 and 11 show signal corresponding to two carbonyl groups.
The reaction of the acid hydrazide 11 with two sugar aldoses namely; D-galactose and D-ribose in presence of catalytic glacial acetic resulted in the formation of the corresponding sugar hydrazones 13 and 14, respectively. The 1H NMR spectra of the afforded sugar hydrazones indicated the absence of the signals assigned for the amino proton and instead the presence of the attributed signals for the hydroxyl protons and the other of the sugar chain signals. The observed chemical shift (δH) assigned for the methine proton (H-1 in the original aldose) and presence of NH signal represents an indication for the formation of the sugar hydrazone possessing the sugar part in its acyclic form (Abdel Aal et al., 2006). For cyclic conformations, the anomeric proton (H-1) in the cyclic glycosyl moiety would appear at lower chemical shift values (El-Sayed et al., 2009).
Cytotoxic activity
The cytotoxic activity of the newly prepared compounds was investigated for against colorectal carcinoma (HCT116), breast adenocarcinoma (MCF7), prostate cancer (PC3) and liver hepatocellular carcinoma (HepG2) human tumor cell lines using the MTT assay (Mosmann, 1983; El-Sayed et al., 2017). The results were formulated (table 1, table 2, Fig. 1 and Fig. 2) as the cytotoxic activity of the synthesized derivatives at 100 μM on the four cell lines under investigation and the corresponding IC50 (μM) values for compounds exhibiting more than 90% at 100 μM (table 3).
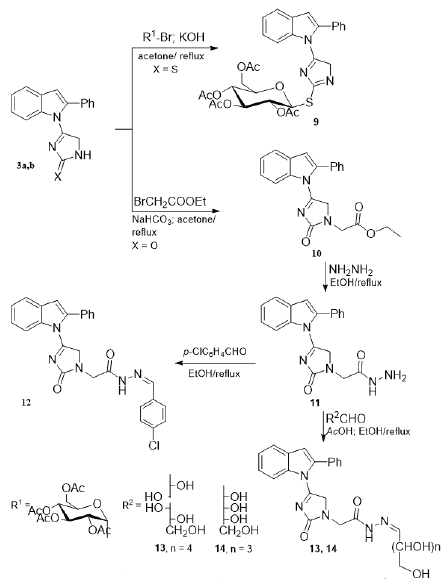 | Scheme 2: Synthesis of indole linked imidazoline and sugar derivatives. [Click here to view] |
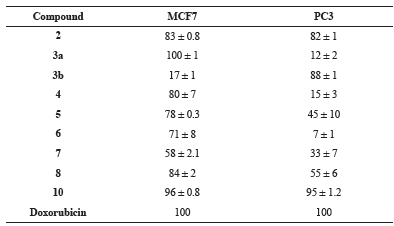 | Table 1: Cytotoxic effect of synthesized compounds at 100 μM on breast adenocarcinoma (MCF7) and prostate cancer (PC3) human tumor cell lines. [Click here to view] |
The afforded screening results proved that compounds 2 and 10 were highly active against all four tested cell lines. Compounds 3a showed a significant cytotoxic effect for cancer cells nearly similar to the reference drug, doxorubicin, against breast adenocarcinoma with IC50 value 1.31 ± 0.8 μM. In addition, such compound also revealed high activity against colorectal carcinoma and liver hepatocellular carcinoma cell lines. On the other hand, compound 10 showed promising activity against breast adenocarcinoma and prostate cancer cell lines in addition to its high cytotoxic effect against colorectal carcinoma and liver hepatocellular carcinoma cell lines. Such compound exhibited IC50 value (7.8 ± 3.3 μM) near to that of doxorubicin against prostate cancer cell lines.
The obtained data from cytotoxicity evaluation against the four cancer cell lines showed that compounds 4-8 showed moderate activity against breast adenocarcinoma cell line. It was also observed that compound 3b, selectively, showed high activity against prostate cancer cell lines.
Trying to constitute a relation of the inhibition activity results with structures of prepared compounds, the obtained results revealed that the attachment of 2-thioxoimidazolyl ring to 2-phenylindole system resulted in increased cytotoxic effect against breast adenocarcinoma, colorectal carcinoma, and liver hepatocellular carcinoma cell lines. Furthermore, functionalization of the imidazolyl-indole system via N1-substitution of the imidazole ring forming the ethyl ester derivative resulted in increased cytotoxicity against the four cell lines. Clearly, the imidazolyl-indole system with oxo-substitution at C-2 in the imidazole moiety showed, interestingly, an obvious enhanced activity more than that of the corresponding thioxo-analogue. Furthermore, linkage of the five-membered imidazole ring to 2-phenylindole resulted in relatively more active derivative than the attachment of the six-membered triazine system. In addition, attachment of functionalized monocyclic five-membered ring moiety revealed higher activity than the linkage of the bicyclic system.
 | Table 2: Cytotoxic effect of synthesized compounds at 100 μM on colorectal carcinoma (HCT116) and liver hepatocellular carcinoma (HepG2) human tumor cell lines. [Click here to view] |
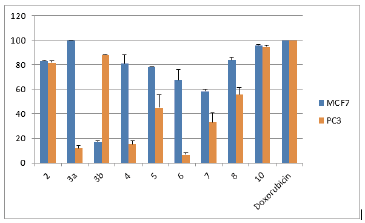 | Fig. 2: The cytotoxic effect of compounds at 100 μM on MCF7 and PC3 human tumor cell lines. [Click here to view] |
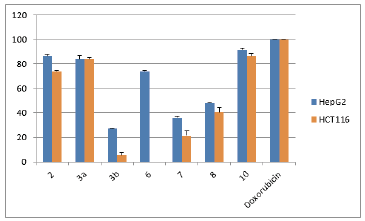 | Fig. 3. The cytotoxic effect of compounds at 100 μM on HepG2 and HCT116 human tumor cell lines. [Click here to view] |
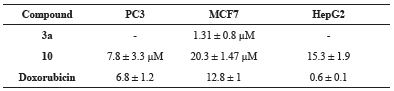 | Table 3: IC50 values (μM) of the compounds which gave more than 90% at 100 μM. [Click here to view] |
CONCLUSION
New indolyl linked imidazole, imidazolothiazole, triazine, thiazolo-s-triazine and imidazole sugar derivatives were synthesized and studied for their cytotoxic activity. A number of compounds showed high activity; one of them incorporating imidazolyl- indole system revealed significant cytotoxic activity against human breast adenocarcinoma. This indicated that the attachment of functionalized imidazolyl ring to the 2-phenylindole core enhanced activities more than triazine and thiazole systems. The results encourage next studies for performing structural modifications of compounds similar to the most active derivatives aiming for enhancing selectivity; e.g. substitution on imidazolyl ring linked to 2-phenylindole for studying the structure-activity relationship toward better cytotoxicity results.
REFERENCES
Abdel Aal MT, El-Sayed WA, El-Ashry ESH. Synthesis and antiviral evaluation of some sugar arylglycinoylhydrazones and their oxadiazoline derivatives. Arch Pharm, 2006; 339:656-663.
Abdel-Rahman AAH, El-Sayed WA, Zaki EG, Mohamed AA, Fadda AA. Synthesis and antimicrobial activity of substituted [(pyrazol-4-yl)methylene]hydrazono-2,3-dihydrothiazoles and their sugar derivatives. J Het Chem, 2012; 49:93-101.
Ali OM, El-Sayed WA, Eid SA, Abdelwahed NAM, Abdel-rahman AAH, Antimicrobial activity of new synthesized [(oxadiazolyl) methyl] phenytoin derivatives. Acta Polan Pham Drug Res, 2012; 69:657-667.
Ballistreri FP, Barresi V, Benedetti P, Caltabiano G, Fortuna CG, Longo ML, Musumarraa G. Design, synthesis and in vitro antitumor activity of new trans 2-[2-(heteroaryl)vinyl]-1,3-dimethylimidazolium iodides. Bioorg Med Chem, 2004; 12:1689-1695.
Bandini M. Electrophilicity: the “dark-side” of indole chemistry. Org Biomol Chem, 2013; 11:5206-5212.
Capon RJ, Peng C, Dooms C. Trachycladindoles A–G: cytotoxic heterocycles from an Australian marine sponge, Trachycladus laevispirulifer. Org Biomol Chem, 2008; 6:2765-2771.
Chan D, Anderson A. Towards Species-specific Antifolates. Curr Med Chem, 2006; 13:377-398.
Cui B, Zheng BL, He K, Zheng, QY. Imidazole Alkaloids from Lepidium meyenii. J Nat Prod, 2003; 66:1101-1103.
D’hooghe M, Mollet K, De Vreese R, Jonckers THM, Dams G, De Kimpe N. Design, Synthesis, and Antiviral Evaluation of Purine-β-lactam and Purine-aminopropanol Hybrids. J Med Chem, 2012; 55:5637-5641.
Dolzhenko AV, Chui W-K. Synthesis of 2-amino-s-triazino[1,2-a]benzimidazoles as potential antifolates from 2-guanidino- and 2-guanidino-5-methylbenzimidazoles. J Het Chem, 2006; 43:95-100.
El-Sayed WA, Abdel-Rahman AAH, Ramiz, MMM. Anti-Hepatitis B Virus Activity of New N4-β-D-Glycoside Pyrazolo [3,4-d] pyrimidine Derivatives. Zeitschrift für Naturforschung, 2009; 64c:323-328.
El-Sayed WA, Abbas HS, Abdel Magid RE, Magdziarz T. Synthesis, antimicrobial activity and docking studies of new N-ethyl-3-indolyl heterocycles. Med Chem Res, 2016; 25:339-355.
El-Sayed WA, El-Sofany WI, Hussein HAR, Fathy NM, Synthesis and Anticancer Activity of New [(Indolyl)pyrazolyl]-1,3,4-Oxadiazole Thioglycosides and Acyclic Nucleoside Analogs. Nucleosides, Nucleotides and Nucleic acid, 2017a; 36:474-495.
El-Sayed WA, Mohamed AM, Khalaf HS, EL-Kady DS, Al-Manawaty M. Synthesis, Docking Studies and Anticancer Activity of New Substituted Pyrimidine and Triazolopyrimidine Glycosides. J Appl Pharmaceut Sci, 2017b; 7:1-11.
El-Sayed WA, Fathi NM, Gad WA, El-Ashry ESH. Synthesis, and Antiviral Evaluation Of Some 5-N-Arylaminomethyl-2-glycosylsulphanyl-1,3,4- oxadiazoles and their analogues against Hepatitis A and Herpes simplex viruses. J Carbohydr Chem, 2008; 27:357-372.
Falcó JL, Piqué M, González M, Buira I, Méndez E, Terencio J, Pérez C, Príncep M, Palomer A, Guglietta A. Synthesis, pharmacology and molecular modeling of N-substituted 2-phenyl-indoles and benzimidazoles as potent GABAA agonists. Europ J Med Chem, 2006; 41:985-990.
Flefel EM, El-Sayed WA, El-Sofany W, Mohamed AM, Awad HM. Synthesis and Anticancer Activity of New 1-Thia-4-azaspiro[4.5]decane, Their Derived Thiazolopyrimidine and 1,3,4-Thiadiazole Thioglycosides. Molecules, 2017; 22:170.
Fortuna CG, Barresi V, Berellini G, Musumarra G. Design and synthesis of trans 2-(furan-2-yl)vinyl heteroaromatic iodides with antitumour activity. Bioorg Med Chem, 2008; 16:4150-4159.
Gangjee A, Jain HD, Kurup S. Recent Advances in Classical and Non-Classical Antifolates as Antitumor and Antiopportunistic Infection Agents: Part I. Anticancer Agents Med Chem, 2007; 7:524-542.
Hu W, Guo Z, Yi X, Guo C, Chu F. Cheng G. Discovery of 2-Phenyl-3-sulfonylphenyl-indole derivatives as a new class of selective COX-2 inhibitors. Bioorg Med Chem, 2003; 11:5539-5544.
Jacquet AB, Lang EG, Malaval AA. Anti-sunburn compositions containing 2-phenyl-indole derivatives. US Patent No. 4,522,808, 1985.
Jennings LD, Foreman KW, Rushi TS, Tsao DHH, Mosyak L, Kincaid SL, Sukhdeo, MN, Sutherland AG, Ding W, Kenny CH, Sabus CL, Liu H, Dushin EG, Moghazeh SL, Labthavikul P, Petersen PJ, Tuckman M, Ruzin AV. Combinatorial synthesis of substituted 3-(2-indolyl)piperidines and 2-phenyl indoles as inhibitors of ZipA–FtsZ interaction. Bioorg Med Chem, 2004; 12:5115-5131.
Johansson H, Jørgensen TB, Gloriam DE, Bräuner-Osborne H, Pedersen DS. 3-Substituted 2-phenyl-indoles: privileged structures for medicinal chemistry. RSC Adv, 2013; 3:945-960.
Kapuscinski J, Skoczylas B. Fluorescent complexes of DNA with DAPI 4′,6-diamidine-2-phenyl indole 2HCl or DCI 4′,6-dicarboxyamide-2-pnenyl indole. Nucleic Acid Res, 1978; 5:3775-3800.
Kompis IM, Islam K, Then RL. DNA and RNA Synthesis: Antifolates. Chem Rev, 2005; 105:593-620.
Li QL, Huang J, Wang Q, Jiang N, Xia CQ, Lin, HH, Wua J, Yu XQ. Monometallic complexes of 1,4,7,10-tetraazacyclododecane containing an imidazolium side: Synthesis, characterization, and their interaction with plasmid DNA. Bioorg Med Chem, 2006; 14:4151-4157.
Miller CP, Tran BD. 3- [4- (2- phenyl-Indole-1-ylmethyl) Phenyl]- acrylamides as estrogenic agents, US Patent No. 5,985,910, 1999.
Miyachi H, Kiyota H, Segawa M. Design, synthesis and antimuscarinic activity of some imidazolium derivatives. Bioorg Med Chem Lett, 1999; 9:3003-3008.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods, 1983; 65:55-63.
Ramla MM, Omar MA, El-Khamry A-MM, El-Diwani HI. Synthesis and antitumor activity of 1-substituted-2-methyl-5-nitrobenzimidazoles. Bioorg Med Chem, 2006; 14:7324-7332.
Shi W, Marcus SL, Lowary TL. Cytotoxicity and topoisomerase I/II inhibition of glycosylated 2-phenyl-indoles, 2-phenyl-benzo[b]thiophenes and 2-phenyl-benzo[b]furans. Bioorg Medi Chem, 2011; 19:603-612.
Toyoda T, Brobey RK, Sano G, Horii T, Tomioka N, Itai A. Lead Discovery of Inhibitors of the Dihydrofolate Reductase Domain of Plasmodium falciparum Dihydrofolate Reductase-Thymidylate Synthase. Biochem Biophys Res Commun, 1997; 235(3):515-519.
Vik A, Hedner, E, Charnock C, Tangen LW, Samuelsen Ø, Larsson R, Bohlinb L, Gundersen LL. Antimicrobial and cytotoxic activity of agelasine and agelasimine analogs. Bioorg Med Chem, 2007; 15:4016-4037.
Walsh JJ, Bell A. Hybrid Drugs for Malaria. Curr Pharm Des, 2009; 15:2970-2985.
Wang R, Shi HF, Zhao JF, He YP, Zhang HB, Liu JP. Design, synthesis and aromatase inhibitory activities of novel indole-imidazole derivatives. Bioorg Med Chem Lett, 2013; 23:1760-1762.
Yousif MNM, El-Sayed WA, Abbas HS, Awad HM, Yousif NM. Anticancer Activity of New Substituted Pyrimidines, Their Thioglycosides and Thiazolopyrimidine Derivatives. J Appl Pharmaceut Sci, 2017; 7:21-32.