INTRODUCTION
The World Health Organization (WHO) defines pharmacovigilance (PV) as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects of drugs. An adverse drug reaction (ADR) is a reaction to a drug and/or a combination of drugs which is harmful and unintended and which occurs at a dose that is normally used for prophylaxis, diagnosis, or treatment” (Aronson, 2012; Coulter, 2013). In 2013, India’s share in globally reported ADRs was only 2%. ADRs reported per million almost doubled in the last few years to 40 but remains far less than in developed nations. Indian regulatory bodies assess the benefit–risk ratio of drugs based entirely on the experience gathered overseas; it has become both urgent and important for India to develop indigenous PV systems. The accumulating evidence suggests that reporting suspected ADRs adds value to PV through the generation of potential signals, and describing potential ADRs in sufficient detail to assess likely causality and reduce the impact of ADRs (Alvarez et al., 2010).
The ADRs entail the unjustified hospital admissions, worsen the hazards from the prevailing shortage of trained health professionals, increase the preventable health hazards, diminish the quality of life, and waste both resources and money. International data revealed that 4.1% of the non-elderly hospital admissions were ADR-related, while it was four times higher for elderly (16.6%) (Beijer and De Blaey, 2002).
A study carried out in south India demonstrated that 0.7% of hospital admissions were due to ADRs, with a total of 3.7% of hospitalized patients experiencing at least one ADR, of which 1.3% were fatal (Ramesh and Parthasarathi, 2009). Another study in India showed that ADRs accounted for 3.4% of the hospital admissions while 3.7% patients developed ADRs during their hospital stay (Arulmani et al., 2008). Yet, another study in Europe showed that 41.2% (42 out of 102) ADRs were preventable if clinical safety surveillance programs were implemented (Dormann et al., 2003).
As newer safety data emerge, safeguarding public health is vital before withdrawing a drug or altering its labeling. Improving the ADR reporting would lead to improved indigenous databases from which we may draw locally relevant safety guidelines. Methods for improving the reporting of ADRs include: (1) early initiation and enforcement of risk management plans by the regulator, (2) education of all those eligible to report, (3) greater transparency of regulatory decisions, and (4) better and quicker dissemination of updates in safety information (Tang, 2010).
The PV and safety monitoring, being barely a decade old in India, are a relatively new concept in this country. On the other hand, the ever-rising number of global clinical trials being conducted in India, along with the increasing number of new drug approvals with the limited drug safety data, together underscores the need for a robust PV system that is in line with international norms (Brahmachari et al., 2011).
Periodic safety update report (PSUR) is defined as “an update of the worldwide safety experience of a medicinal product submitted to competent authorities at defined times post-authorization. According to regulatory norms, the marketing authorization holders (MAHs) must prepare PSURs and make them available to the regulatory authority” (Amy Tang, 2010).
It is important to note that all major drugs were being sold in the Indian market at the time of withdrawal. Indian regulatory bodies have been relying on the experience gathered overseas to assess the benefit–risk ratio of a drug, making it imperative for India to develop its own PV system. High profile drug withdrawals that drew the attention of Drugs Controller General of India (DCGI) and WHO alike, highlighted the inadequacy of PV systems implemented by MAHs in India (Arora, 2012).
Schedule Y of Drug and Cosmetics Act 1945 stipulates legislative requirements for PV in India. Following multiple incidents of high-profile drug withdrawals, Schedule Y was thoroughly reviewed and amended in January 2005 to ensure adequate PV compliance by the MAHs. Schedule Y contains a section on post-marketing surveillance with guidelines and requirements for PSUR cycle, a template for PSUR, PSUR submissions, and the timelines for expedited reporting. The revised Schedule Y recommends that “for all new products, PSURs should be submitted every 6 months for the first 2 years and thereafter annually for the next 2 years”. Schedule Y has adopted the same format of PSUR as given in ICH E2C (Arora, 2012).
A previous study noted that PSUR evaluations contributed to 38% of post-authorization regulatory actions in a sample of bio-pharmaceuticals (Ebbers et al., 2012). In addition, in 2010, another study observed that 64% of a selection of ADRs originated from PSURs (Alvarez et al., 2010).
Reports of myocardial infarctions and strokes in patients taking Vioxx® resulted in its withdrawal from the market in 2004. Likewise, Lipobay® (Baycol®; cerivastatin), a hypolipidemic drug; fenfluramine and dexfenfluramine, an anti-obesity drug in addition to many others, were voluntarily withdrawn by MAHs due to safety reasons. Experts have suggested that only a probationary license should be given for a new drug, which should be confirmed only when drug post-marketing studies demonstrate acceptable risk–benefit balance (Greener, 2005). The latest data from the website of central drug standard control organization (CDSCO), the Indian drug regulatory authority, indicate that around 90 drugs or their combinations were withdrawn from the market because of their low-safety profile (CDSCO, 2009).
Though rules in India mandate that after launching a drug, its side-effects, fatalities, and injuries in Indian patients need to be documented, MAHs often do not comply with the guidelines (Sinha, 2012). When the Indian government constituted a parliamentary standing committee to scrutinize the compliance of PSUR submissions, the CDSCO could supply only eight reports from a randomly selected 42 newly introduced drugs. The standing committee report says: “The committee takes strong exception to such rampant violation of the mandatory requirements. The committee recommends that the Union Health Ministry should direct the CDSCO to send a stern warning to all manufacturers of new drugs to comply with PSURs or face suspension of marketing approval. The committee is of the firm view that there is a poor follow-up of side-effects in Indian patients both by doctors and MAHs” (Indian parliamentary standing committee report, 2013).
After the parliamentary standing committee reported on the issue, CDSCO, in August 2012, amended the guidelines for mandatory submission of PSURs for all the MAHs within the given timeline. The CDSCO also issued orders to a few major hospitals in the country to submit PSURs for all drugs launched since 2011 and are being prescribed in the respective hospitals (CDSCO, 2013).
The Delphi method is a useful way of discovering and determining uncertainties and has been widely exploited in medical and health services (Akins, 2005). The Delphi method is a collaborative expert system where the experts are provided with a Delphi design and where they dynamically and actively contribute their knowledge to the system. This method significantly broadens the knowledge and effective decision making in health and social care (Hasson et al., 2000; Shariff, 2015).
To measure opinions, perceptions, and behaviors, Likert scales are the most preferred among others. This method let us reveal the degree of outlook that possibly constructs a real difference in understanding the feedback. It can also give a better understanding of areas where you need to focus more and improve the process and function. Compared to binary questions, which give you only two answer options, it can help decide whether you strongly agree or strongly disagree with any system or process (Survey monkey, 2018).
In this context, we decided to design a system for implementing the PSUR reporting system in all wards of our hospital in accordance with the regulatory requirement. We also evaluated the quality of functioning of PSUR system implemented in our hospital with the help of a PSUR function assessment questionnaire (PFAQ), which was subsequently validated by a Delphi panel, before it was circulated among the healthcare practitioners (HCPs). Test samples of three highly prescribed drugs, with already known ADRs, were chosen to evaluate the functioning of PSUR system. On the other hand, the impact of PSUR system implementation was evaluated by comparing the frequency and rate of ADR reporting for selected three drugs before and after the implementation of PSUR system.
MATERIALS AND METHODS
Implementation of PSUR system in the hospital
Ethical permission was obtained from the institutional ethics committee of the hospital before starting the study (Ref: IEC 195/2013). A PSUR committee was formed in January 2013 to carry out ADR documenting, preparation, and submission of PSURs. Drug information leaflets, ADR reporting guidelines, and ADR reporting forms were prepared and circulated in all wards of a hospital for manual ADR reporting. ADR reporting software was also developed and linked with the intranet website of the hospital for online reporting of ADRs. Appropriate training on the basics of ADR, manual and online ADR reporting for newer drugs under PSUR system was provided to HCPs. Hands-on training on a medical dictionary for regulatory activities (MedDRA) coding software, Vigiflow and hospital information services software (hospitals internal patient management and billing software) were provided to Pharm D interns and students.
Evaluation of the impact of PSUR system implementation using validated PFAQ
The quality of system implementation was evaluated using a duly validated questionnaire vetted by a Delphi panel of 10 members consisting of in-house clinical pharmacist, chief nurse/pharmacist, physician, and faculty members from Department of Pharmacy Practice. Success factors and performance indicators listed in PFAQ were successively added or deleted by the Delphi panel, in two successive rounds of revision, yielding the final draft of PFAQ. Final PFAQ draft lists 11 success factors and 8 performance indicators based on which 88 questions were listed in the final PFAQ form. The sample size for two proportions (responses on PFAQ for the particular time, phase 1 and phase 2) was calculated to 40 respondents in each phase considering 500 HCPs population size in a hospital with a confidence interval of 95% and margin of error 15%. Final PFAQ was circulated among HCPs (physicians, nurses, pharmacists, interns, and Pharm D students) to rate their responses on the Likert scale of 1–5 where 1 corresponds to “very good” and 5 corresponds to “very poor.”
Evaluation of the impact of PSUR system implementation and functioning using safety surveillance of three highly prescribed drugs
On account of paucity of data, it was not possible to judge the quality and extent of ADR reporting of newer drugs. Therefore, changes in the pattern, frequency, and rate of ADR reporting for three widely prescribed drugs, namely, amlodipine, furosemide, and risperidone were taken as a proxy to the impact of PSUR system implementation. It was necessary to choose drugs with well-known ADR profiles in published literature to be considered reliable markers of the impact of PSUR system implementation. The study period lasted 18 months, divided into two intervals, namely 9 months before and 9 months after PSUR system implementation. The ADR data were collected from the medical records retrospectively for the first 9 months before the PSUR system was implemented. After the HCPs (physicians, nurses, pharmacists, interns, and Pharm D students) were trained and familiarized with the system of PSUR, the ADR data for the above drugs were again captured and compared with the 9 months post-PSUR system implementation. The differences in ADRs were captured in terms of number, category, frequency, rates, and locations where ADRs were encountered.
RESULTS
Evaluation of the impact of PSUR system implementation using validated PFAQ
 | Table 1. Preliminary list of success factor’s and performance indicator’s circulated in first review round of PFAQ validation. [Click here to view] |
 | Table 2. Updated list of success factor’s and performance indicator’s circulated in second review round of PFAQ validation. [Click here to view] |
The preliminary draft PFAQ contained 19 items (10 success factors and 09 performance indicators) for evaluation and it was sent out to all 10 panel members in round 1 of the Delphi process. The first review round of PFAQ (Table 1) item validation was initiated on July 1, 2014. The responses on PFAQ from all the panel members were completed and received on August 20, 2014. All 19 items mentioned in PFAQ draft were answered and received in the first review round. Expert panel members reached 70% consensus to include (aggregate percentage of Likert scales 1 and 2) seven success factors and eight performance indicators in PFAQ. However, panel also reached to 70% consensus to exclude (aggregate percentage of Likert scale 4 and 5) three success factors (latest drug list, reporting of IP numbers, and patient follow-up) and one performance indicator, practicability. Delphi panel also suggested considering some additional success factors (drug dispensing database, wards/departments, nursing station, and pharmacy) and performance indicator (overall feedback) to be included in the second review round of PFAQ. The updated list of 20 items (success factors and performance indicators) based on first review responses for PFAQ was circulated for second review round to Delphi expert panel on September 1, 2014 (Table 2). Delphi panel provided responses to all the PFAQ items on October 31, 2014. In the second review round, 70% consensus was achieved for seven success factors to include them in final PFAQ and to exclude three success factors (drug brands, safety review, and periodic review and analysis) and one performance indicator (relevance). Additionally, there have been strong proposal by Delphi panel to include four more success factors (safety review/consultation, review and analysis of data, ADR data, and PSUR news-letter) in the final PFAQ.
Total 88 questions were framed and included in final PFAQ based on the validated success factors and performance indicators vetted by Delphi panel during first and final review rounds. The consensus was considered reached if at least 70% of the expert panel members strongly agree or disagree that success factors and performance indicators should be included or excluded in the final PFAQ. The questions were framed for each 11 success factors to assess their success for implemented PSUR system using eight validated performance indicators (11 success factors × 8 performance indicators = 88 questions) shown in Table 3. Responses to each question were recorded on a Likert scale of 1–5 where 1 corresponds to very good and 5 corresponds to very poor. The structure of PFAQ with the examples of few questions included in PFAQ are given below in Table 4.
One hundred sixty PFAQs were circulated in the different wards of hospitals from November 15, 2014 until May 16, 2016. Total 94 filled PFAQs responses were received until May 16, 2016. The PFAQ responses were recorded in two phases of 9 months interval each. The PFAQs received in the first 9 months were kept in phase 1 (November 15, 2014 to August 15, 2015) and remaining PFAQs received in last 9 months were kept in phase 2 (August 16, 2015 to May 16, 2016). There were 46 responses received in phase 1 and 48 responses in phase 2 from HCPs.
Responses on success factor, overall assessment of the functioning of PSUR system reflect the success of PSUR implementation which indicates there is a significant improvement in the performance indicators rated by HCPs during the phase 2 study period compared with phase 1 study phase. Feedback by HCPs on overall assessment seen shifted favorably in phase 2 study period (68.7%) on the Likert scale 1 (very good) and 2 (good) compared with phase 1 (50%) as shown in Figure 1.
Evaluation of the impact of PSUR system implementation and functioning using safety surveillance of three highly prescribed drugs
The results presented here have been compiled from the data spread across 18 months (March 2013 to August 2014) both before and after implementation of PSUR system. In these 18 months, a total of 2,827 patients were prescribed amlodipine, furosemide, or risperidone in the hospital. ADRs reported for the above three drugs rose from 65 in the first 9 months (pre-PSUR) to 107 during the subsequent 9 months (post-PSUR). Overall, there was a 68% rise in ADR reporting rate from the different wards of hospitals for selected three drugs after the implementation of PSUR system.
Of the reported 172 ADRs for the 18 months study period, 30 ADRs were reported from amlodipine, 102 ADRs from furosemide, and 40 ADRs were reported from risperidone. All these reported ADRs for study duration were assessed for their severity using Hartwig severity scale. Thompson and Rawlins ADR classifications method was used to assess types of ADRs. Naranjo scale was used to assess causality of ADRs. Seriousness, outcome, and length of hospitalization increased due to ADRs were also assessed as shown in Table 5. Overall, there were 8.1% ADRs were found to be severe in nature, while 55.2% ADRs were analyzed as mild in severity. Severity of reported ADRs was found to be on higher side prior to PSUR system implementation (severe 5.1%; moderate 26%). Seven (7) out of 172 ADRs were categorized to Type B reactions (idiosyncratic). Of the seven reported type B ADRs, four ADRs were reported prior PSUR system implementation. Totally, 11 (6.39%) patients were reported to have life threatening conditions of which five patients were reported to have life threatening conditions prior to PSUR system implementation. Increased in length of hospitalizations due to ADRs were experienced by 32 (18.6%) patients of which 10% patients have been identified post-PSUR implementation. There were 14 ADRs assessed to have probable associations between the event and study drug; most of these ADRs (6%) were reported post-PSUR system implementation. The outcome of 11 ADRs (6 ADRs reported prior PSUR implementation) were reported as fatal while 15 ADRs (all ADRs reported post PSUR implementation) were still continuing while the ADRs have been recorded. The average increase in hospitalization due to ADRs was recorded to be 3 days.
 | Table 3. Validated success factors and performance indicators by Delphi panel included in final PFAQ. [Click here to view] |
Implementation of PSUR system raised the ADR reporting rates for amlodipine (from 2% to 4.62%), furosemide (5.69% to 9%), and risperidone (from 6.12% to 10%). The overall rate of ADR reporting rose from 4.60% to 7.87% post-PSUR system implementation (Tables 6 and Fig. 2).
The frequency of ADR reporting increased manifold post-PSUR system implementation. For instance, reports of pedal oedema in amlodipine users increased from 40% to 70% after PSUR implementation (Fig. 3). Although ADRs for amlodipine were reported from many wards like psychiatry, medicine, and cardiology; the maximum increase (75%) came from medicine ward (Fig. 4).
Similarly, there was a significant rise in reports of hyponatremia (28.5% to 45.1%) and hypokalemia (27.5% to 50%) with furosemide post-PSUR system implementation (Fig. 5). This is possibly because of increased awareness and alertness about this clinically significant category. ADR reporting on furosemide from nephrology and cardiology wards increased significantly from 5% to 37% and 25% to 29%, respectively (Fig. 6).
Likewise, ADR reports for risperidone from psychiatry ward increased from 6.12% to 10% post-PSUR system implementation. Of these, nine ADRs were never reported before PSUR were implemented. Reports of extrapyramidal symptoms and tremors rose from 13.3% to 16% and 33.3% to 36%, respectively, post-PSUR, indicating greater awareness of important complications of therapy (Fig. 7).
DISCUSSIONS
This is among the first studies conducted in a health care setting in India to assess the process, functioning, and implementation of drug safety reporting network in a tertiary care hospital. Therefore, there is a lack of data available from similar studies to compare the outcomes of this study. We have discussed here the outcomes along with indicators of the selection process carried out via Delphi methods and perception of HCPs toward ADR reporting, which have been reported in other developed countries.
There are not any specific guidelines suggesting what should be the ideal Delphi panel size require carrying out Delphi survey because panel size is purposely selected and it depends on the problem being investigated. Delphi survey studies do not represent the panel size to be used in terms of statistical purposes; therefore, sample size determination for Delphi panel has completely different approach than other surveys (Shariff, 2015). Thus, we selected optimum panel size of 10 members to be involved in two review rounds of success factors and performance indicators validation and PFAQ finalization.
 | Table 4. Structure of final PFAQ based on validated success factors and performance indicators. [Click here to view] |
Likert scale was used in this study to validate performance indicators and success factors and finalize the PFAQ via Delphi panel. Also, it was requested from HCPs to rate their responses on the Likert scale in PFAQ on implemented drug safety network and PSUR system in the hospital. We have observed the responses received on Likert five-point rating scales gives more power to concerned query compared with binary questions, which only gives two answer options. Likert scale helps to decide HCPs whether they are strongly agree or strongly disagree with any system or process (Alshakka et al., 2013; Survey Monkey, 2018).
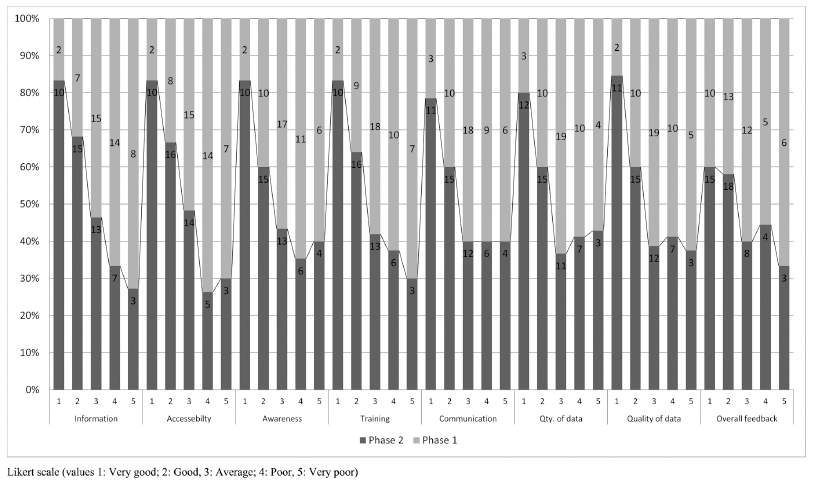 | Fig 1. Overall assessment of Implemented PSUR system in different wards of hospital via PFAQ responses recevied during the phase 1 and phase 2 study duration. [Click here to view] |
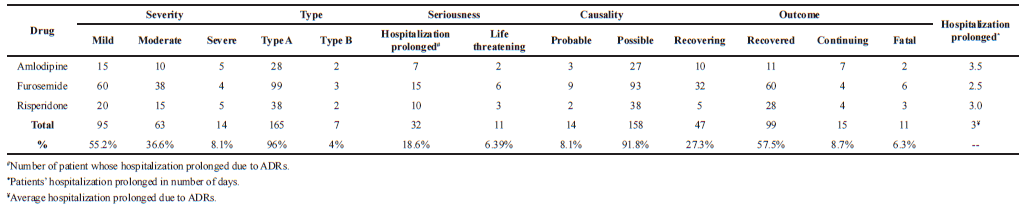 | Table 5. Assessment of ADRs for their severity, type, seriousness, causality, length of hospitalization and outcome. [Click here to view] |
The responses received to validate success factors and performance indicators to finalize PFAQ via Delphi panel were found to be higher 58.75% in our study in contrast to similar study carried out in Malaysia, where only 18% panel members participated to determine the perception of physicians toward ADR reporting (Alshakka et al., 2013).
Despite, many national and international drug safety reporting schemes are in place, we are far behind in reporting of ADRs. One systematic study carried out in Europe suggests that the lack of knowledge, time, interest, and uncertainty about causality is the major cause in under-reporting of ADRs (Hopf et al., 2016). Few government and privately funded agencies have found out that, two-thirds of the 411 (65.8%)respondents had no knowledge of the ADR reporting system. A very small percentage of respondents 101 (16.2%) had ever reported ADRs in their professional career. This kind of studies justifies why such drug safety reporting network and training platforms should be implemented in each hospital to improve the rate of ADR reporting in the hospital (Mulatu, 2014). Even results of the final PFAQ survey among HCPs reveals that, HCPs are more informed (52%), have better accessibility (54.1%), more aware (52%), have gone through appropriate training (56.2%), have better communication with PSUR team and work station (54.1%), and there is an overall improvement (68.75%) in the drug safety reporting and monitoring system during the phase 2 study period since the PSUR system implemented in our hospital (phase 1) as shown in Figure 1.
ADR reporting rate for selected three drugs from published literatures in Indian health-care setup was found to be in the range of 3% to 6% for amlodipine, 5% to 10% for furosemide, and 3% to 10% for risperidone (Aqil et al., 2006; Khurshid et al., 2012; Lihite et al., 2017; Piparva et al., 2011). A similar pattern of ADR reporting was found in our study for selected drugs as well (Fig. 2). Prior to 6-month ADR reporting study carried out at medicine ward of our hospital reveals total 317 (22%) ADRs were reported from 1,438 patients (Thiagu, 2011). This finding contradicts the result outputs of our study reported from the same hospital. We found the ADR reporting rate for selected three drugs was 4.6% prior to PSUR system implemented in the hospital and rose to 7.87% post-PSUR implementation. The reason behind the difference in the ADR reporting rate might be due to, we had selected pool of patients, who were prescribed either of three selected drugs. Though, we have observed there is a significant increment in ADR reporting rate (75%) for amlodipine from medicine ward and furosemide (35%) from Nephrology ward since PSUR system implementation in the hospital (Fig. 4).
One systematic meta-analysis study carried out at Portugal endorses our work by suggesting that the projects included in their study which were using active promotional/interventional methods for ADR reporting have doubled the rate of ADR reporting compared with other projects which were using passive promotion of ADR reporting methods (Vaz et al., 2016a; 2016b). A similar study was conducted in Malaysia to find out the impact of knowledge intervention on community pharmacist in ADR reporting. It was observed in the study, 76% participants were unaware of pharmacovigilance and ADR reporting. It has been found out in the study, ADR reporting rate among the surveyed pharmacists was very less (9%) and ADR reporting rate was found to be significantly increased after their participation in the study (Elkalmi et al., 2011). The similar trend of increment of ADR reporting rate observed in our study for selected three drugs when routed through well-administered PSUR system (Fig. 2).
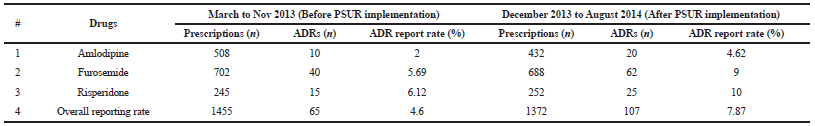 | Table 6. ADR reporting rate pre and post PSUR system implementation. [Click here to view] |
One related study of perception on ADR reporting among physicians, in government and private hospitals in Kuwait, reveals that the private physicians demonstrated a better knowledge of PV basics (75.8% vs. 65.3%; p = 0.001) and practice (75.2% vs. 64.8%; p = 0.002) (Alsaleh et al., 2017). A similar study carried out in Kolkata, India found that 92% physicians in hospital settings believed that reporting ADRs is necessary and would benefit the patient. While, 74% physicians have a belief that ADR reporting is a professional obligation for doctors. That means, HCPs are aware about ADR monitoring and reporting process but the lack of implementation of such program of drug safety reporting network in health care setup resist them to report ADRs (Kamtane et al., 2012).
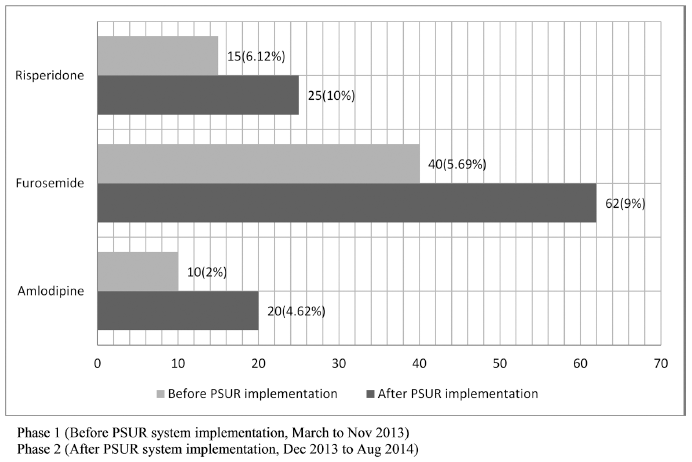 | Fig 2. ADR reporting rate pre and post PSUR system implementaion for selected three highly prescribed drugs, risperidone, furosemide and amlopdipine. [Click here to view] |
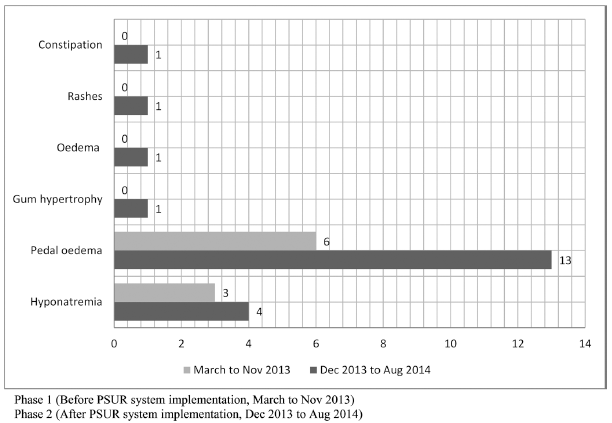 | Fig 3. Rate of ADR reporting by MedDRA preferred terms pre and post PSUR system implementation for Amlodipine. [Click here to view] |
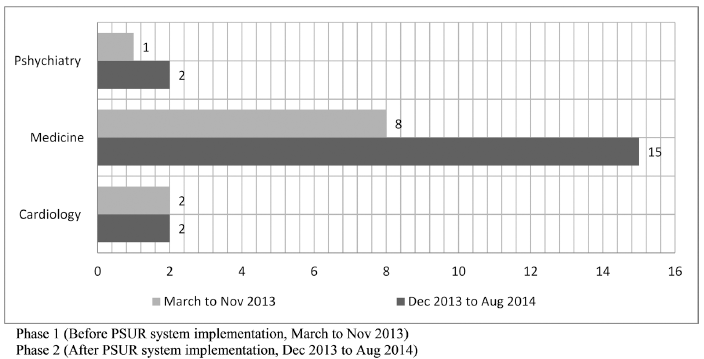 | Fig 4. ADR reporting by Ward pre and post PSUR system implementation for Amlodipine. [Click here to view] |
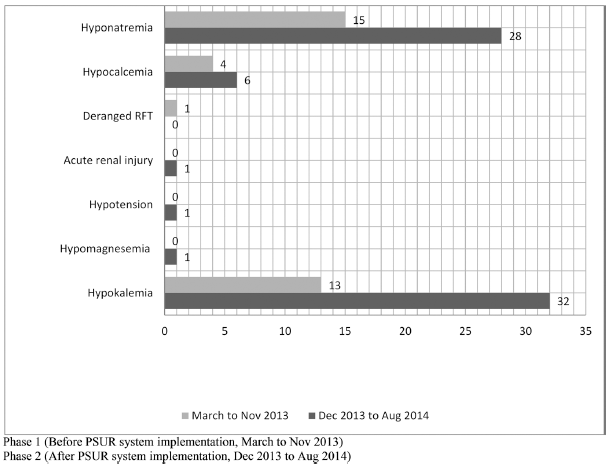 | Fig 5. Rate of ADR reporting by MedDRA preferred terms pre and post PSUR system implementation for furosemide. [Click here to view] |
 | Fig 6. ADR reporting by Ward pre and post PSUR system implementation for furosemide. [Click here to view] |
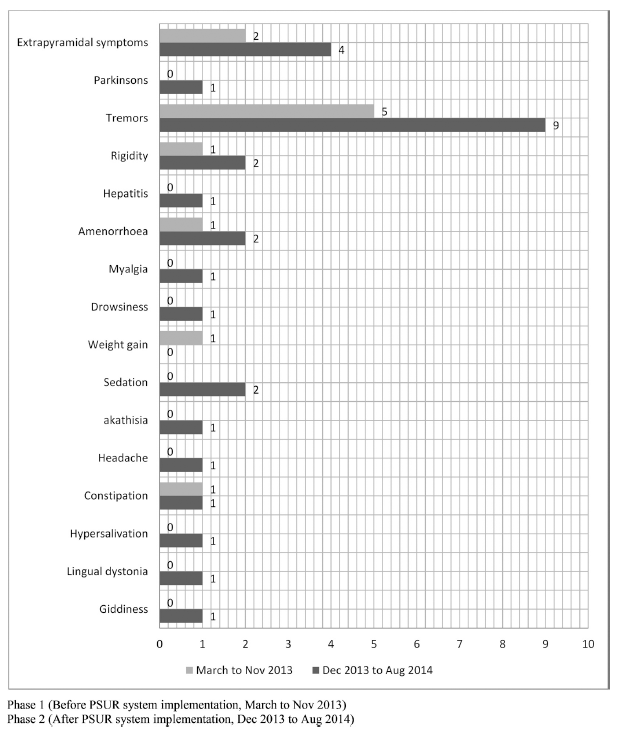 | Fig 7. Rate of ADR reporting by MedDRA preferred terms pre and post PSUR system implementation for risperidone in Psychiatry ward. [Click here to view] |
CONCLUSION
The current study supports the critical importance of implementing drug safety network and periodic safety update reporting systems, particularly in Indian hospitals. We observed that the rate of ADR reporting increased by 68% from different wards of the hospital for the test samples when ADR monitoring was conducted by trained HCPs and routed through a well-administered PSUR system. All the wards of the hospital have been sensitized and alerted toward ADR reporting.This study illustrates how a formal drug safety reporting network can be established by redeploying the existing resources without additional capital investments/human resources in training or new job positions. Implementation of PSUR system in the teaching hospital has greater long-term benefits because it enhances participation of all players across the healthcare team, including students. Being linked to the educational program, such a system also helps spread awareness among the HCPs on ADR reporting and analysis.PSUR system implementation also increases awareness about the incidence, severity, causality, preventability, and risk–benefit ratio of medications. Reporting unexpected/unreported ADRs is critically important for ethnically diverse countries such as India, which does not generate many clinical data because of fewer formal clinical trials and even fewer new drug launches.
PSUR reporting can generate accurate drug safety surveillance data and assist DCGI in taking decisions on safety-related issues of newer drugs and validate the accuracy of drug safety data (PSURs) submitted by MAHs. The PSUR data submitted by MAHs is inaccessible and confidential because pharmaceutical industries are reluctant to disclose results which adversely impact their marketed products. PSURs generated by teaching hospitals are less likely to be tainted by conflicts of interest and will provide more accurate data accessible to all including the lay public. The dissemination of PSUR through newsletters, publications, and so on adds value to the exercise.STUDY LIMITATIONS
There were only three drugs selected to assess the impact of newly implemented PSUR systems in terms of ADR reporting rate pre- and post-PSUR system implementation; which couldn’t predict the success of implemented PSUR system in the hospital. However, later, 11 drugs launched in the year 2011 in India were included for ADR reporting under this PSUR system and we would be presenting further study results from this program shortly.
ACKNOWLEDGMENTS
We wish to thank all the healthcare professionals including students and interns for their undue support for this PSUR project implementation and functioning in the hospital. We also thank the Kasturba hospital, Manipal administration for providing all the support. Our sincere thanks to DCGI office for this initiative and providing necessary training to carry out this research work.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
Nil.
REFERENCES
Akins R. A process-centered tool for evaluating patient safety performance and guiding strategic improvement. Agency Healthcare Res Qual, 2005; (4):109–25.
Alsaleh FM, Lemay J, Al Dhafeeri RR, AlAjmi S, Abahussain EA, Bayoud T. Adverse drug reaction reporting among physicians working in private and government hospitals in Kuwait. Saudi Pharm J, 2017; 25(8):1184–93.
Alshakka MA, MMI, Hassali MAA. Do health professionals have positive perception towards consumer reporting of adverse drug reactions? J Clin Diagn Res, 2013; 7(10):2181–5.
Alvarez Y, Hidalgo A, Maignen F, Slattery J. Validation of statistical signal detection procedures in eudra vigilance post-authorization data: a retrospective evaluation of the potential for earlier signalling. Drug Saf, 2010; 33(6):475–87.
Amy T. Evaluating the evidence base in pharmacovigilance decision making, 2010. Available via http://eprints.port.ac.uk (Accessed 13 March 2013).
Aqil M, Imam F, Hussain A, Alam M, Kapur P, Pillai K. A pharmacovigilance study for monitoring adverse drug reactions with antihypertensive agents at a South Delhi hospital. Int J Pharm Pract, 2006; 14(4):311–3.
Aronson JK. Stephens’ detection and evaluation of adverse drug reactions: principles and practice. 6th edition, John Willy and Sons, NJ, 2012.
Arora D. Pharmacovigilance obligations of the pharmaceutical companies in India. Indian J Pharmacol, 2012; 40(1):2–9.
Arulmani R, Rajendran SD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol, 2008; 65(2):210–6.
Beijer HJM, Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci, 2002; 24(2):46–54.
Brahmachari B, Fernandes M, Bhatt A. Pharmacovigilance for clinical trials in India: current practice and areas for reform. Perspect Clin Res, 2011; 2(2):49–53.
CDSCO. List of drugs prohibited for manufacture and sale. 2009. Available via http://cdsco.nic.in/writereaddata/drugs banned in thed country.pdf (Accessed 25 March 2015).
Coulter D. A practical handbook on the pharmacovigilance of antiretroviral medicines. WHO Press, World Health Organization, Geneva, Switzerland, 2013.
Dormann H, Rieck CM, Neubert A, Egger T, Geise A, Krebs S, Schneider TH, Levy M, Hahn EG, Brune K. Lack of awareness of community-acquired adverse drug reactions upon hospital admission: dimensions and consequences of a dilemma. Drug Saf, 2003; 26(5):353–62.
Ebbers HC, Teeuwisse MAK, Moors EHM, Tabatabaei SFA, Schellekens H, Leufkens HGM. A cohort study exploring determinants of safety-related regulatory actions for biopharmaceuticals. Drug Saf, 2012; 35(5):417–27.
Elkalmi RM, Hassali MA, Ibrahim MIM. Impact of educational intervention for improving pharmacist knowledge in adverse drug reactions (ADR) reporting: experience from Malaysia. Open Drug Saf J, 2011; 2:47–53.
Fifty-ninth report on the functioning of the central drugs standard control organisation (CDSCO). 2013. Available via http://164.100.47.5/newcommittee/reports/EnglishCommittees/Committee on Health and Family Welfare/59.pdf (Accessed 26 April 2015).
Greener M. Drug safety on trial. EMBO Re, 2005; 6(3):202–4.
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs, 2000; 32(4):1008–15.
Hopf YM, Francis J, Helms PJ, Haughney J, Bond C. Linking NHS data for pediatric pharmacovigilance: results of a Delphi survey. Res Social Adm Pharm, 2016; 12(2):267–80.
Kamtane RA, Jayawardhani V. Knowledge, attitude and perception of physicians towards adverse drug reaction (ADR) reporting: A pharmacoepidemiological study. Asian J Pharm Clin Res, 2012; 5(3):210–4.
Khurshid F, Aqil M, Alam MS, Kapur P, Pillai KK. Monitoring of adverse drug reactions associated with antihypertensive medicines at a university teaching hospital in New Delhi. DARU J Pharm Sci, 2012; 20(1):1.
Sinha K. Post-marketing periodic safety update reports of drugs seldom adhere to norms, 2012. Available via https://timesofindia.indiatimes.com/india/Post-marketing-periodic-safety-update-reports-of-drugs-seldom-adhere-to-norms/articleshow/13089140.cms (Accessed 13 May 2013).
Lihite RJ, Lahkar M, Das S, Hazarika D, Kotni M, Maqbool M, Phukan S. A study on adverse drug reactions in a tertiary care hospital of Northeast India. Alexandria J Med, 2017; 53(2):151–6.
Mulatu NW. Assessment of knowledge, attitude and practice of health professionals towards adverse drug reaction reporting and factors associated with reporting. J Pharmacovigil, 2014; 2(4):1–7.
Piparva K, Chandrani K, Buch J. Analysis of adverse drug reactions of atypical antipsychotic drugs in psychiatry OPD. Indian J Psychol Med, 2011; 33(2):153.
Ramesh M, Parthasarathi G. Adverse drug reactions reporting: attitudes and perceptions of medical practitioners. Asian J Pharm Clin Res, 2009; 2(2):10–4.
Vaz RI, Santos CC, Correia CR. Promoting adverse drug reaction reporting: comparison of different approaches. Rev Saúde Pública, 2016a; 50(0):1–9.
Vaz RI, Silva AM, Santos CC, Correia CR. How to promote adverse drug reaction reports using information systems – a systematic review and meta-analysis. BMC Med Inform Decis Mak, 2016b; 16(1):27.
Shariff NJ. Utilizing the Delphi survey approach: a review. J Nurs Care, 2015; 4(3):1–6.
Thiagu R. Modeling of predictors for adverse drug reactions and Pharmacoeconomic Impact in atertiary care hospital, 2011. Available via http://shodhganga.inflibnet.ac.in/bitstream/10603/2398/13/13_chapter 4.pdf (Accessed 13 March 2013).
What is a Likert scale? 2018. Available via https://www.surveymonkey.com/mp/likert-scale (Accessed 1 January 2018).