INTRODUCTION
Fluoroquinolones (FQs) constitute an important group of antibacterial agents as they have numerous applications in the treatment of many bacterial infections in humans, especially those affecting the urinary tract and respiratory tract. They are also effective for the treatment of a broad spectrum of animal infections. They are effective against Mycobacterium, Gram-negative, anaerobes, and Gram-positive bacteria. The mechanism by which FQs exert their bactericidal effect is through the inhibition of bacterial topoisomerases, specifically DNA gyrase and topoisomerase IV. Bacterial DNA replication consists of three distinct stages: initiation, elongation, and termination. Initiation occurs at the commencement of replication, while elongation involves the movement of replication forks outward until they meet halfway around the chromosome. Termination marks the separation of the daughter chromosomes that were previously connected together. These enzymes are essential for all three phases. FQs have a wide safety margin as they have a minute effect on inhibiting human enzymes [1,2]. An FQ antibiotic called ciprofloxacin hydrochloride (CPF) (Fig. 1) is used to treat infections in the lungs (pneumonia) and blood (septicemia) that are caused by Pseudomonas aeruginosa. In addition, it exhibits efficacy against Enterobacteriaceae, the causative agents of wound infections, urinary tract infections, gastroenteritis, meningitis, and pneumonia. In addition, CPF demonstrates efficacy against Moraxella catarrhalis, the pathogenic agent responsible for otitis media in infants, acute exacerbations of chronic obstructive pulmonary disease, and acute bacterial rhinosinusitis [2].
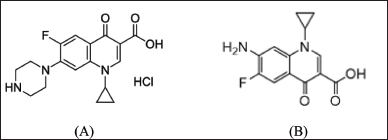 | Figure 1. Chemical structure of CPF (A) and its main photo-degradation product (B). [Click here to view] |
Photo-stability of drugs refers to how light affects the stability of medicinal components and/or final products. As a result, photo-stability examines the way in which light influences the stability of the medication molecules. Light-sensitive drugs can be affected by either natural or artificial light sources. Sunlight may cause liaisons between the medication’s molecule and endogenous materials, transforming the medication into dangerous unwanted products that may result in the generation of numerous oxygenated free radicals. Even a modest quantity of light exposure can have serious effects on the photosensitive drugs; thus, it is not always necessary to expose them to light extensively. The formation of phototoxic substances eventually affects the efficacy of CPF as a light-sensitive medication. Simultaneously, the therapeutic potency is diminished by photodegradation [3].
The measurement of active pharmaceutical ingredients in the presence of their degradants is a vital part of quality control for each medication, notably in the case of FQs, which are known to be susceptible to photo, acidic, alkaline, oxidative, and thermal degradation procedures [4]. The primary degradant of CPF following subjecting its aqueous acidic solution to either artificial or daylight was 7-amino-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid [main photo-degradate (MDP)]. After more than 5 hours of CPF treatment with a high-pressure mercury lamp, it was still the dominant product. MDP exhibits lower therapeutic efficacy and can induce phototoxic reactions [5]. Figure 1 depicts the chemical formulae of CPF and its principal photo-degradant (MDP). In a variety of sample forms, CPF was quantified using spectroscopy [6–8], liquid chromatography (LC) [9–13], capillary electrophoresis [14,15], and electrochemistry [16–18]. Numerous methods were employed to determine the drug under investigation’s stability in the presence of its numerous products of degradation. Attia et al. [19] quantified CPF in the presence of the product of its acid-catalyzed degradation using a variety of spectrophotometric methods, including ratio difference, ratio derivative, mean centering, and dual-wavelength. Aksoy et al. [20] applied high-performance LC (HPLC) to quantify the investigated drug in solid dosage form to determine its stability. Bushra et al. [21] used the same analytical method to measure CPF in the presence of its major photo, basic, acidic, and oxidative degradants. Furthermore, HPLC was utilized to assess the stability of CPF when it was in combination with other pharmaceuticals such as tinidazole [22,23], dexamethasone [24], and metronidazole [25].
Ion-selective electrodes (ISEs) provide several benefits, including simplicity in preparation, rapid response, selectivity, the flexibility to use turbid analyte solutions, and low cost. In addition, they respond without regard to the surface area of the sensor, enabling electrode downsizing [26,27]. ISEs were used for the potentiometric determination of CPF in a variety of sample formats, either alone [28–30] or in conjunction with other medications [31,32].
In ISEs with liquid contact, internal solution evaporation results in a change in the volume of the internal solution as well as an osmotic pressure variation. Pressure fluctuations cause water to move across the membrane, which leads to membrane damage. To get around the difficulties associated with liquid contacting the inner electrodes, solid contact electrodes, which do away with the use of the inner filling solution in favor of a junction of solid state between the metal electrode and the membrane, have lately gained popularity. Solid contact electrodes in CPF analysis have the advantages of miniaturization, simplicity in design, and low cost if compared with conventional liquid contact electrodes [33].
About 50%–70% of the administered CPF dose is excreted unchanged in urine and released from the sewage treatment plant outlet into aquatic ecosystems, posing a growing environmental hazard. The broad and widespread utilization of FQs, particularly CPF, to treat various human and animal infections presents a potential environmental danger. As a result, the occurrence of these chemicals in waterways, where compost or sludge is deposited into soils, or where hospital effluent is discharged into the adjacent stream cannot be ruled out [34]. The long-term exposure of surface water to sunlight may increase the chance for the photo-degradation of photo-sensitive drugs like FQs.
After a comprehensive review of the literature, it was found that there are currently no publications for the potentiometric photo-stability indicating the determination of the researched drug in the presence of its primary photo-degradant.
The main task of this work is to fabricate, optimize, and validate potentiometric solid-contact glassy carbon membrane electrodes for the quantification of CPF in the presence of its main photo-degradant (MPD) and the application of the constructed sensors for the reliable determination of CPF in river water.
EXPERIMENTAL
Instrumentation
The potentiometric measurement was carried out by a Jenway (UK) mV/pH meter, which is attached to an Ag/AgCl reference electrode. A 3 mm-diameter glassy carbon electrode (CH Instruments, Bee Cave, TX) was used to make the solid contact. It is also used as a support for the fabricated membranes and to conduct the generated membrane potential.
Chemicals, reagents, and standard solutions
Pure materials of CPF (CAS # 93107-08-5) and MDP (CAS # 105674-91-7) with percentage purities of 100.78 and 100.92, respectively, were obtained from Cymit Quimica S.L. (Barcelona, Spain). A group of chemicals comprising NaOH, hydrochloric acid, tetrahydrofurane, KCl, phosphotungesic acid (PTA), and tetraphenyl borate (TPB) was purchased from Prolabo (France). On the other hand, bis(2-ethyl hexyl) sebacate (2-EHS), nitrophenyl octyl ether (NPOE), polyvinyl chloride (high molecular weight), and dioctyl phthalate (DOP) were supplied from Sigma (Germany). The deionized water was obtained from Aquatron water still.
In deionized water, a CPF stock standard solution (1 × 10−2 M) was prepared. Through judicious dilution with the same solvent, stock CPF was utilized for preparing working standard solutions in the concentration range of 1 × 10−3 to 1 × 10−8 M.
Sensors’ fabrication, calibration, and optimization
In two different Petri plates, PVC (190 mg), 2-EHS (0.4 ml), and TPB (TPB-sensor) or PTA (PTA-sensor) (10 mg) were combined. In 6 ml of THF, the mixtures were dissolved. The THF solution was introduced directly to a glassy carbon electrode. It allowed for the applied solvent to entirely evaporate. The produced membrane underwent conditioning by soaking for 24 hours in a 0.01 M CPF stock standard solution. The same CPF solution was used to store the electrodes.
After the conditioning phase, the produced sensors were calibrated by dipping them in CPF standard solutions (1 × 10−2–1 × 10−8 M). Each membrane was washed in between measurements with deionized water. The standard graphs were plotted. Then, these figures were used to determine the unknown CPF concentrations.
Evaluation and modification of the factors affecting membrane performance resulted in the best membrane performance. According to the IUPAC’s recommendations, the electrodes’ competence was assessed [35]. To evaluate how the nature of the plasticizer affected the membrane’s working behavior, the author tried three different plasticizers (DOP, 2-EHS, and NPOE) with different polarities to find the one that produced the best electrode performance. By the dropwise addition of sodium hydroxide or hydrochloric acid at a concentration of 0.1 M to a CPF standard solution (1 x 10-4 M), the effect of pH on the performance of the membranes was examined. A graph relating the potential of the examined electrode against the solutions’ pH was drawn to identify the ideal working pH. The reproducibility of the CPF sensors and their potential stability were observed over the course of a month. On a daily basis, the potential was measured using CPF standard solutions at concentrations ranging from 1 × 10−2 to 10−6 M, and the slope (mV/decade) for each membrane was computed. The estimated slopes were compared to the original calibration slopes.
The separate solution method (SSM) was used to evaluate membrane selectivity [36]. The potential responses of the built electrodes in the presence of various interferents, such as inorganic ions (Na+, K+, and NH4+) and structurally related FQs (moxifloxacin hydrochloride and gatifloxacin hydrochloride), were used to calculate the potentiometric selectivity coefficient (PSC) for each interferent. The potential responses were obtained for 0.001 M CPF, and the potential of the same interferent concentration was measured in a separate way. The PSCs were then calculated with the aid of the following formula:
PSC = (E1 − E2)/S
where “E1” is the measured potential for the 0.001 M CPF solution, “E2” is the measured potential for the 0.001 M interferent solution, and “S” is the slope of the corresponding membrane.
Application
Quantification of CPF in the presence of its MDP
Different laboratory-prepared blends containing intact CPF (90%–10%) and its primary photo-degradant (10%–90%) were prepared by combining complementary amounts of CPF and its MPD. The prepared combinations’ pH levels were adjusted to lie between 2.0 and 5.0. The prepared membranes were dipped in the prepared mixtures, and the obtained potentials were used to get the CPF concentration using the calibration graphs. The recovery percentages were computed by dividing the obtained CPF concentrations from the calibration curves by the added CPF concentrations in the prepared mixtures, then multiplying by 100.
Determination of CPF in different water samples
A number of distilled and tap water samples were prepared with definite CPF concentrations. The pH of the prepared samples was set in the range of 2.0–5.0. The drug concentrations were quantified using the cited sensors, and the recovery percentage was computed.
Three CPF-free river water samples were taken at three different locations along the Nile in Cairo, Egypt. The samples’ pH was set to be between 2.0 and 5.0. To remove tiny particle debris, filters made of nylon were applied to filter the samples. To prevent sample degradation, the filtered solutions were kept in dark vials made of glass. Calculated amounts of CPF pure material were dissolved in the CPF-free river water samples to reach CPF concentrations of 1 × 10−3 M, 1 × 10−4 M, and 1 × 10−5 M. Using standard curves, the manufactured membranes were utilized to quantify CPF concentrations, and the recovery percentage was computed based on the initial spiked concentrations.
RESULTS AND DISCUSSION
Due to the wide applicability of CPF in veterinary and human medicine, there is a high probability of its presence in the environment, especially in surface water like river water. The long-term exposure of the river water to sunlight and photo-liability of CPF may increase the photodegradation of CPF. From this perspective, the fabrication of membrane-sensitive electrodes is an important task to monitor, evaluate, and quantify CPF in the presence of its MPD. Potentiometric membrane sensors are used alongside other analytical techniques because they are simple to use and inexpensive, and they have the merits of high sensitivity, no sample pre-processing procedures, and ease of downsizing [37].
The art of the study is demonstrated by the solid-contact potentiometric measurement of CPF using TPB and PTA as ion-pairing agents in a PVC matrix. Ion exchange across the constructed sensors determines the observed potential, which is proportional to the concentration of CPF in its solution.
Based on the mentioned art of the work, this study presents the design of two membrane electrodes with selective sensitivity for quantifying CPF in the presence of its primary photo-degradant in river water without the requirement for sample pre-processing.
Evaluation and validation of the fabricated membranes
The evaluation parameters declaring the performance efficiency of the fabricated electrodes are shown in Table 1. In the concentration range of 1 × 10−6 and 1 × 10−2 M CPF, an essentially optimal Nernstian behavior was obtained. The slopes of sensors 1 and 2 were 60.1 ± 0.70 and 57.9 ± 0.90 mV/decade, respectively (Table 1). Figure 2 displays typical graphs.
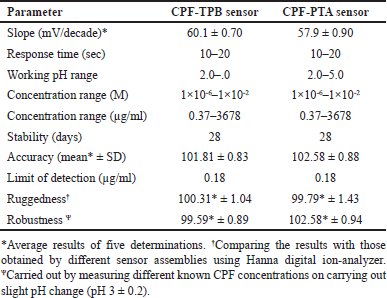 | Table 1. Electrochemical response characteristics of the fabricated electrodes. [Click here to view] |
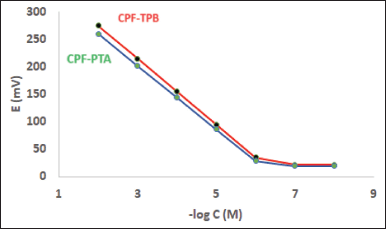 | Figure 2. Profile of the potential (in mV) versus–log concentration (in M) for CPF sensors at pH 3. [Click here to view] |
The fabricated membranes’ performance was assessed using IUPAC guidelines [35], including response time, ideal pH range, linearity, plasticizer type influence, and detection limit.
The effect of plasticizer type was explored by testing three plasticizers with varying polarity: 2-EHS, NPOE, and DOP. The one that produced the best results was 2-EHS. The membranes fabricated using this plasticizer display closer slopes to the ideal Nernstian ones. This is due to the comparable polarity of the investigated medication and the plasticizer. The CPF and 2-EHS combination secured the best durability and enabled the most efficient ion exchange through the fabricated sensors, but the membranes made from NPOE and DOP exhibited poor non-Nernstian slopes, which are 50.20 ± 0.30 (n = 3) and 49.80 ± 0.70 mV/decade (n = 3), respectively. The sensitivity of the fabricated sensors was evaluated by defining the detection limit at the point of intersection of the standard curve linear segments [37]. Detection limits as low as 0.18 µg/ml exhibited excellent sensitivity (Table 1). The detection limits of the developed sensors demonstrate their exceptional sensitivity, which is appropriate for their use in environmental analysis. They also displayed a fast response during the analysis of a wide range of concentrations. The mentioned parameters constitute the most vital ones for the sensitive quantification of CPF in real-environmental samples when applying the developed membranes.
The medium pH affects the operation of the sensors. The pH range is 2.0 to 5.0 (Fig. 3). A significant deviation of the calibration curves from linearity was observed when the pH values exceeded five. Because of the drug’s decreased solubility. A non-Nernestian behavior was noticed when the pH of the test solution exceeded the value of 5.
By plotting calibration graphs and daily assessing their attributes, the membranes’ lifetimes were estimated to be 28 days (Fig. 4). An important parameter, which is the response time, plays an important role in the process of membrane evaluation. It shows the required time for the fabricated sensor to provide a constant potential value (±1 mV) after a ten-fold concentration increase [35]. The response time of the built sensors was discovered to be 15 seconds.
Assay accuracy was evaluated by applying the fabricated sensors for the determination of CPF samples with definite concentrations. The analytical assay’s ability to withstand deliberate changes in working conditions (minor pH alterations) was examined for method robustness. Furthermore, the procedure’s ruggedness was evaluated to ascertain its reproducibility. Table 1 summarizes these parameters.
The SSM [36] was used to assess membrane selectivity, where various inorganic interferents, including Na+, K+, and NH4+, and structurally related interferents (moxifloxacin hydrochloride and gatifloxacin hydrochloride), were tested and the PSC for each interferent was calculated. Table 2 displays the calculated PSCs. The small values of the calculated PSCs may confirm the method’s high selectivity. The lipophilicity of the sample ion, the interferent ion on the sample side, and the three-dimensional (3D) structure all affect how select the cited method is. It is also affected by the 3D structure of the receptor of the ion exchanger. Sensor selectivity is influenced by the sensor constituents, the type of solvent used, and the ratio of plasticizer to PVC. Lipophilic ionic sites increase interfacial ion-exchange kinetics. TPB and PTA are ionic sites that have been introduced to membranes used to measure cations to improve selectivity and sensitivity. TPB or PTA are captured by the positively charged CPF nitrogen during the preconditioning stage until an equilibrium condition is reached and the sensor is adequate to determine the researched drug ion. Membrane sensors require plasticizers that enable rapid ion motion and good physical characteristics for the fabricated membrane [38].
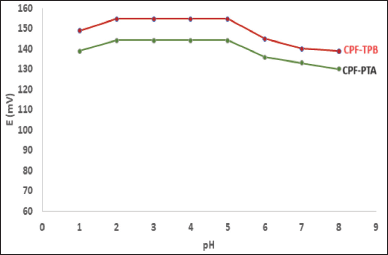 | Figure 3. pH effect on the potential response of CPF membrane sensors. [Click here to view] |
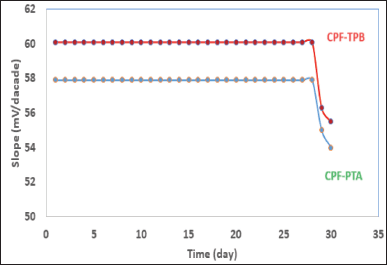 | Figure 4. Stability of the CPF membrane sensors. [Click here to view] |
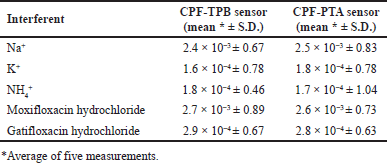 | Table 2. PSCs of the proposed CPF selective sensors by the SSM. [Click here to view] |
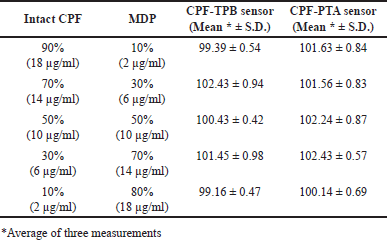 | Table 3. Results obtained for the analysis of laboratory-prepared mixtures containing different ratios of intact CPF and its MDPs using the fabricated sensors. [Click here to view] |
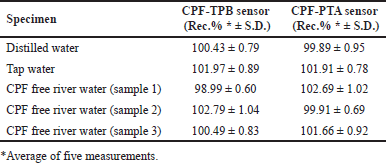 | Table 4. Determination of CPF in spiked river water samples using the fabricated membrane sensors. [Click here to view] |
Method application
Determination of CPF in the presence of its MDP product
Investigating the method selectivity was carried out in the presence of up to 90% CPF MPD. To perform this task, the sensors were used to analyze a variety of laboratory-prepared mixtures containing 9 × 10−4 M–1 × 10−4 M CPF and 1 × 10−4 M–9 × 10−4 M MDP. The results shown in Table 3 reveal the selectivity of the suggested method and confirm the capability of the assay to quantify the cited drug in the presence of up to 90% MDP, where the deviations from the 100% recovery did not exceed 2.5% up and 1% low.
Assay of CPF-loaded water samples
The sensors were successfully utilized to quantify CPF in either distilled or tap water samples containing the medication of interest. In addition, the sensors were used to analyze many CPF-free river water samples loaded with known CPF concentrations. This was done to validate the sensors’ performance across different water matrices. Table 4 shows the calculated recovery percentages.
CONCLUSION
The originality of the work arises from the absence of prior research on the potentiometric assessment of the target drug in the presence of its principal photo-degradant, which is commonly found in river water containing CPF owing to the long-term exposure to sunlight and its ease of photo-degradation. The presence of MDP in the river water samples containing CPF constitutes a major challenge for its quantification due to the high chemical similarity between CPF and its MPD. The absence of a sample pre-treatment procedure verifies the method’s versatility in comparison to the spectroscopic and chromatographic methods utilized for CPF analysis. Furthermore, the suggested approach is more economical than UPLC and LC-MS/MS since it has fewer expenses per sample. The electrochemical performances of the constructed sensors are equivalent. They are effectively applied in a wide concentration range and at different pH levels. Both membranes have been used effectively for the sensitive and accurate assessment of CPF in river water samples.
FINANCIAL SUPPORT
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2023/03/25359).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Sweetman SC. Martindale: the complete drug reference. 36th ed. London: The Pharmaceutical Press; 2009.
2. Da Silva AD, De Almeida MV, De Souza MV, Couri MR. Biological activity and synthetic methodologies for the preparation of fluoroquinolones, a class of potent antibacterial agents. Curr Med Chem. 2003;10(01):21–39. CrossRef
3. Hayashi N, Nakata Y, Yazaki A. New findings on the structure-phototoxicity relationship and photostability of fluoroquinolones with various substituents at position 1. Antimicrob Agents Chemother. 2004;48(03):799–803. CrossRef
4. Maia AS, Ribeiro AR, Amorim CL, Barreiro JC, Cass QB, Castro PML, et al. Degradation of fluoroquinolone antibiotics and identification of metabolites/transformation products by liquid chromatography–tandem mass spectrometry. J Chromatogr A 2014;1333:87–98. CrossRef
5. Torniainen K, Mattinen J, Askolin CP, Tammilehto S. Structure elucidation of a photodegradation product of ciprofloxacin. J Pharm Biomed Anal. 1997;15(07):887–94. CrossRef
6. Pandey S, Pandey P, Tiwari G, Tiwari R, Rai AK. FTIR spectroscopy: a tool for quantitative analysis of ciprofloxacin in tablets. Indian J Pharm Sci. 2012;74(01):86–90. CrossRef
7. Cazedey ECL, Salgado HRN. Spectrophotometric determination of ciprofloxacin hydrochloride in ophthalmic solution. Adv Anal Chem. 2012;2(06):74–79.
8. Qassim AW. Spectrophotometric determination of ciprofloxacin hydrochloride in pharmaceutical formulation ciproxin. Int J Adv Sci Tech Res. 2015;3(05):135–46.
9. Tsanaktsidou E, Markopoulou CK, Tzanavaras PD, Zacharis CK. Homogeneous liquid phase microextraction using hydrophilic media for the determination of fluoroquinolones in human urine using HPLC-FLD. Microchem J. 2022;172:106906. CrossRef
10. Wei L, Chen Y, Shao D, Li J. Simultaneous determination of nine quinolones in pure milk using PFSPE-HPLC-MS/MS with PS-PAN nanofibers as a Sorbent. Foods. 2022;11:1843. CrossRef
11. Maddeppungeng NM, Mir M, Raihan M, Wahyudin E, Asma N, Layadi P, et al. Application of quality by design approach in HPLC-UV validation of ciprofloxacin in porcine ocular tissue and simulated ocular fluid for quantification in an ex vivo ocular kinetic study. Chem Data Collect. 2022;41:100900. CrossRef
12. Haidari-Khoshkelat L, Raoof JB, Ghani M, Ojani R. Combination of in-situ electro synthesized Zn–Al-LDH@ pencil graphite fiber and three phase hollow fiber LPME for microextraction of some antibiotics in urine samples and quantification via HPLC-UV. Anal Chim Acta 2022;1235:340532. CrossRef
13. Tantawy MA, Wahba IA, Saad SS, Ramadan NK. Two validated chromatographic methods for determination of ciprofloxacin HCl, one of its specified impurities and fluocinolone acetonide in newly approved otic solution. J Chromatogr Sci. 2022;60(07):655–62. CrossRef
14. Ko?ka I, Purgat K, Kubalczyk P. Simple, fast and reliable CE method for simultaneous determination of ciprofloxacin and ofloxacin in human urine. Sci Rep. 2022;12(01):7729. CrossRef
15. Abdel-Gawad SA, Altharawi A. Simultaneous quantification of some fluoroquinolone residues in real wastewater effluents using CZE. Separations. 2023;10:292. CrossRef
16. Hosseini A, Sohouli E, Gholami M, Sobhani-Nasab A, Mirhosseini SA. Electrochemical determination of ciprofloxacin using glassy carbon electrode modified with CoFe2o4-MWCNT. Anal Bioanal Electrochem. 2019;11(08):996–1008.
17. Azriouil M, Matrouf M, Ettadili FE, Laghrib F, Farahi A, Saqrane S, et al. Recent trends on electrochemical determination of antibiotic Ciprofloxacin in biological fluids, pharmaceutical formulations, environmental resources and foodstuffs: direct and indirect approaches. Food Chem Toxicol. 2022;168:113378. CrossRef
18. Pollap A, Baran K, Kuszewska N, Kochana J. Electrochemical sensing of ciprofloxacin and paracetamol in environmental water using titanium sol based sensor. J Electroanal Chem. 2020;878:114574. CrossRef
19. Attia KAM, Nassar MWI, El-Zeiny MB, Serag A. Stability-indicating methods for the analysis of ciprofloxacin in the presence of its acid induced degradation product: a comparative study. Spectrochim Acta A. 2016;159:219–22. CrossRef
20. Aksoy B, Küçükgüzel ?, Rollas S. Development and validation of a stability-indicating HPLC method for determination of ciprofloxacin hydrochloride and its related compounds in film-coated tablets. Chromatographia. 2007;66(01):57–63. CrossRef
21. Bushra MU, Huda MN, Mostafa M, Sultan MZ, Rahman A. Study of forced degradation of ciprofloxacin HCl indicating stability using RP-HPLC method. Der Pharma Chem. 2013;05:132–37.
22. Jansari SK, Patel NB, Patel PR, Patel NN, Desai HT. Development and validation of stability indicating method for simultaneous estimation of Ciprofloxacin HCl and Tinidazole using RP-UPLC method. IOSR J Pharm. 2013;2(05):12–19. CrossRef
23. Vaghela BK, Rao SS. A novel validated stability indicating high performance liquid chromatographic method for estimation of degradation behavior of ciprofloxacin and tinidazole in solid oral dosage. J Pharm Bioallied Sci. 2013;5(04):298–308. CrossRef
24. Razzaq SN, Ashfaq M, Mariam I, Khan IU, Razzaq SS, Mustafa G. Stability indicating RP-HPLC method for simultaneous determination of ciprofloxacin and dexamethasone in binary combination. J Chil Chem Soc. 2017;62(03):3572–77. CrossRef
25. El-Zaher AA, Elkady EF, El-Hakim MM, Mandoor A. Stability indicating HPLC method for the simultaneous determination of ciprofloxacin hydrochloride and metronidazole in the presence of ciprofloxacin acid degradation product. Asian J Biochem Pharm Res. 2015; 1(5):5–17.
26. Stradiotto NR, Yamanaka H, Zanoni MVB. Electrochemical sensors: a powerful tool in analytical chemistry. J Braz Chem Soc. 2003; 14:159–73. CrossRef
27. Abdel-Gawad SA, Arab HH. Potentiometric sensors for the selective determination of benzodiazepine drug residues in real wastewater effluents. Chemosensors. 2022;10:74. CrossRef
28. Ahmed AMK, Hanoon IT, Ahmed SH. Determination of the ciprofloxacin hydrochloride drug in some pharmaceuticals using manufactured membrane selective electrodes. Syst Rev Pharm. 2020;11:622–26.
29. Rezk MR, Riad SM, Khattab FI, Marzouk HM. Electrochemical determination of ciprofloxacin hydrochloride in pharmaceutical formulation, aquatic environ-ment and in fish tissues. Int J Biol Pharm Res. 2013;4:390–96.
30. Abdel-Haleem FM, Rizk MS, Badr IH. Potentiometric determination of ciprofloxacin in physiological fluids using carbon paste and nano-composite carbon paste electrodes. Electroanalysis. 2017;29(4):1172–79. CrossRef
31. Santos AM, Wong A, Almeida AA, Fatibello-Filho O. Simultaneous determination of paracetamol and ciprofloxacin in biological fluid samples using a glassy carbon electrode modified with graphene oxide and nickel oxide nanoparticles. Talanta. 2017;174:610–18. CrossRef
32. Sakur AA, Dabbeet HA, Noureldin I. Novel drug selective sensors for simultaneous potentiometric determination of both ciprofloxacin and metronidazole in pure form and pharmaceutical formulations. Res J Pharm Technol. 2019;12(7):3377–84. CrossRef
33. Schwarz J, Trommer K, Mertig M. Solid-contact ion-selective electrodes based on graphite paste for potentiometric nitrate and ammonium determinations. Am J Anal Chem. 2018;9:591–601. CrossRef
34. Lombardo-Agüí M, Gámiz-Gracia L, García-Campaña AM, Cruces-Blanco C. Sensitive determination of fluoroquinolone residues in waters by capillary electrophoresis with laser-induced fluorescence detection. Anal Bioanal Chem. 2010;396(4):1551–57. CrossRef
35. Lindner E, Umezawa Y. Performance evaluation criteria for preparation and measurement of macro-and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl Chem. 2008;80:85–104. CrossRef
36. Ma TS, Hassan SM. Organic analysis using ion selective electrodes. Academic Press: London, UK, 1982.
37. Salem AA, Barsoum BN, Izake EL. Potentiometric determination of diazepam, bromazepam, and clonazepam using solid contact ion-selective electrodes. Anal Chim Acta. 2003;498:79–91. CrossRef
38. Abdel-Gawad SA, Arab HH, Albassam AA. Potentiometric determination of moxifloxacin by solid-contact ISEs in wastewater effluents. Chemosensors. 2022;10:146. CrossRef