INTRODUCTION
Curcuma xanthorrhiza Roxb (Family Zingiberaceae) is a rhizomatous plant originates from Indonesia. Locally known as “temulawak,” C. xanthorrhiza is cultivated in all major islands in Indonesia and throughout Southeast Asia regions, including Malaysia, the Philipines, Vietnam, and Sri Lanka. Traditionally, rhizomes are widely recognized for the treatment of various health conditions, such as liver and digestive problems, infection, fatigue, rheumatism, and lack of appetite [1]. Therefore, they have become an important part of jamu formulation since ancient times [2].
Various bioactive compounds have been identified in the rhizomes of C. xanthorrhiza, such as terpenoids, curcuminoids, and phenolics [3]. Of these, xanthorrhizol (XTZ) is most abundantly isolated, especially from the rhizome oil of C. xanthorrhiza [4]. XTZ (chemical name: 2-methyl-5-[(2R)-6-methylhept-5-en-2-yl]phenol) is a phenolic bisabolane sesquiterpenoid and appears as a colorless oil with an aromatic ring and a side chain with isoprene and hydroxyl groups (Fig. 1). XTZ was also identified in the rhizomes of other Curcuma species, such as C. aromatica [5], C. longa [6], C. aeruginosa [7], and C. angustifolia [8].
Different in vitro and in vivo studies have shown that XTZ possesses diverse pharmacological activities such as antioxidant, anti-inflammatory, antimicrobial, anticancer, and protective activities on important organs (Fig. 1). This review provides essential information focusing on the bioactivities of XTZ that are particularly isolated from C. xanthorrhiza. Its cellular and molecular mechanisms underlying these effects are studied. However, the present study did not discuss the anticancer activities of XTZ, which have already been discussed in our previous paper [9]. Based on the findings of this literature study, future opportunities for investigation on XTZ are discussed.
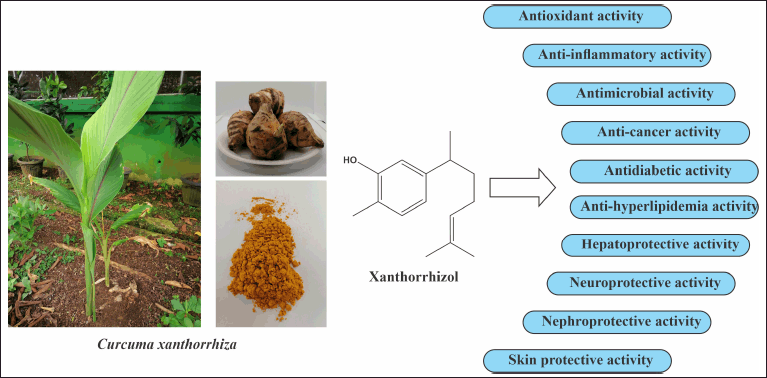 | Figure 1. XTZ and its health benefits. [Click here to view] |
METHODS
The literature review aimed to provide an overview of the different pharmacological activities of XTZ obtained from C. xanthorrhiza. A search strategy was designed to obtain relevant publications, primarily searched using PubMed and ScienceDirect databases from inception through December 2021. The keywords used were “bioactivities AND xanthorrhizol,” “bioactivities AND Curcuma xanthorrhiza,” “antibacterial AND xanthorrhizol,” “antibacterial AND Curcuma xanthorrhiza,” “antidiabetic AND xanthorrhizol,” “antidiabetic AND Curcuma xanthorrhiza,” antioxidant AND xanthorrhizol,” “antioxidant AND Curcuma xanthorrhiza,” “inflammatory AND xanthorrhizol,” “inflammatory AND Curcuma xanthorrhiza,” “Toxicity AND xanthorrhizol,” “toxicity AND Curcuma xanthorrhiza.” The search results were then screened by the following criteria. Only original articles published in English were included in the study. Cancer studies related to XTZ and C. xanthorriza were excluded, as this topic has been covered in the previous review by our group. According to this criteria, 63 publications were explored for extraction.
Antioxidant and anti-inflammatory activity of XTZ
It is well known that reactive oxygen species (ROS) are the main cause of oxidative damage. Under normal metabolic pathways, the body generates ROS through the cellular oxidation process. A low level of ROS serves a key role as redox signaling molecules to induce cell biological processes. However, high ROS generation, such as from external exposure to environmental pollutants, can cause damaging modifications of different biomolecules (lipid, protein, and DNA). In addition, indirectly, ROS can activate inflammatory pathways. Transcription factor nuclear factor-kappa (NFκB) is known to be redox-sensitive and can be activated to induce gene expression of inflammatory proteins [10]. Persistent oxidative stress may amplify inflammation, leading to chronic inflammatory conditions. This condition has been associated with the progression of various degenerative diseases, such as cancer, metabolic diseases, and neurodegenerative diseases. To date, various studies have described the antioxidant and anti-inflammatory activities of XTZ and C. xanthorrhiza and the molecular mechanisms of these effects.
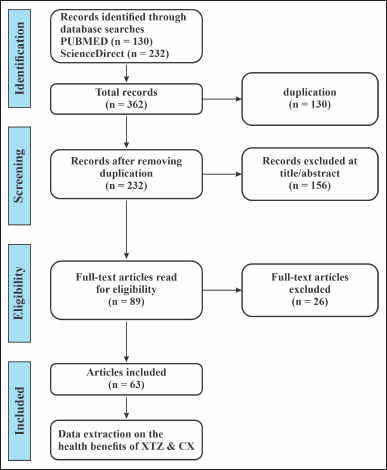 | Figure 2. Article screening applied for the literature search of the health benefits of XTZ and C. xanthorrhiza (CX). [Click here to view] |
Antioxidant activity of XTZ
Different methods were used to evaluate the antioxidant activity of C. xanthorrhiza and XTZ. In general, results showed that XTZ has stronger antioxidant activity than the rhizomes extracted with different solvents [11]. Further, the antioxidant activity of the rhizomes depends on the solvents used for the extraction, indicating that different compounds in the rhizomes contribute to the activity [12]. Awin et al. [13] found that the total phenolic content, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and NO radical scavenging activities of C. xanthorrhiza are influenced by the preparation of the rhizomes and the extractants. The freeze-dried rhizomes had the strongest inhibition and highest concentration of phenolics compared with oven-dried and air-dried rhizomes [13]. Ethanol was better in extracting antioxidant compounds from C. xanthorrhiza compared to aqueous ethanol (IC50 of 26.8 and 82.0 μg/ml, respectively, in the DPPH assay) [13]. In another study, Jantan et al. [14] found that methanol extract of C. xanthorrhiza inhibited peroxidation of human LDL more strongly (IC50 of 0.78 μg/ml), compared to that of essential oil (IC50 of 2.2 μg/ml).
Other plant parts of C. xanthorrhiza also show good antioxidant activity. In studies using the flower bract, methanol, and ethyl acetate extracts showed good DPPH scavenging activity (IC50 6.60 and 12.68 μg/ml, respectively); however, weak inhibitions were observed for hexane and the essential oil (both of >200 μg/ml) [15]. XTZ separated from methanol extract also showed good DPPH scavenging activity (IC50 > 16 μg/ml) [15].
Curcuma xanthorrhiza oil supplementation could be beneficial in increasing the activity and production of endogenous antioxidant enzymes in poultry. In heat challenge poultry, a diet with C. xanthorrhiza supplementation was able to increase the activities and the mRNA levels of superoxide dismutase (SOD) and catalase (CAT) in the heart, liver, and kidney and reduced the mRNA level of heat shock protein [11].
XTZ, as the biomarker of C. xanthorrhiza, has also been studied for its antioxidant activity against lipid peroxidation. XTZ was found to have comparable antioxidant activity to probucol against the oxidation of human LDL, with IC50 0.4 and 0.30 μg/ml, respectively [14]. XTZ at 10 μM was also found to entirely inhibit the H2O2-induced lipid peroxidation of the rat brain homogenate [16].
XTZ showed a neuroprotective effect against glutamate-induced oxidative stress in murine hippocampal HT22 cell lines. At concentrations 1 and 10 μM, treatment with XTZ suppressed ROS generation in HT22 cells dose-dependently.
In a study using human neuroblastoma SK-N-SH cells, XTZ exhibited radical scavenging activity against 4-hydroxynonenal (HNE), a lipid peroxidation product. The increased level of HNE was associated with the aggregation of amyloid β (Aβ) peptides in Alzheimer’s disease. XTZ treatment in SK-N-SH cells decreased the modification and inactivation of neprilysin [17], an important protease in the degradation of Aβ.
Generally, it was accepted that phenolic antioxidants, including XTZ, exert their radical scavenging activity by single electron transfer, followed by proton transfer of the hydroxylic group to produce phenolic radical cation. The side chain in the XTZ structure may participate in stabilizing phenolic radical cation, thus contributing to the scavenging activity. However, Lim et al. [16] confirmed that the hydroxyl group of XTZ is key to the scavenging activity. It was found that acetylation of the −OH group resulted in the loss of antioxidant activity of XTZ [16]. Ichikawa et al. [18] reported that the position of the −OH group was essential to the antioxidant activity. Curcuphenol, an isomer of XTZ, showed stronger DPPH radical scavenging activity than XTZ since the OH group of curcuphenol is closer to the side chain [18].
Anti-inflammatory activity of XTZ
Inflammation is a protective body response against microbial invasion and injury in its cells and tissues. Controlled inflammation response is important in the clearance of inflammatory stimuli, thus restoring normal physiological functions. Failure to control inflammation leads to chronic inflammation conditions, autoimmune response, and excessive tissue damage [19].
XTZ and C. xanthorrhiza have demonstrated anti-inflammatory activities by in vitro and in vivo models using different cells, as shown in Table 1. They inhibit inflammation by suppressing the production of cytokines, proinflammatory enzyme mediators cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), and transcription factors. Studies also reported their action in downregulating signaling pathways involved in inflammation, as summarized in Figure 3.
Cytokines are signaling proteins secreted in the host cells in response to inflammation. Suppression of proinflammatory cytokines is key in regulating inflammation [20]. XTZ and C. xanthorrhiza exert their anti-inflammatory activity by suppressing the production of proinflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α) in in vitro and in vivo models [21–23]. XTZ also suppressed the mRNA expression of matrix metalloproteinase (MMP)-2 and MMP-8, which are an important class of cytokines [21].
Several studies also reported the ability of XTZ to suppress the protein expression of COX-2 and iNOS in cell systems [16,24]. Both enzymes are important inflammatory mediators These activities were also reported using animal models of TPA-induced skin inflammation mice [25] and in dextran sulphate sodium (DSS)-induced colitis mice [22]. XTZ was also demonstrated to be a potent inhibitor of COX-2 and iNOS, with IC50 of 0.2 and 1 ug/ml, respectively [24].
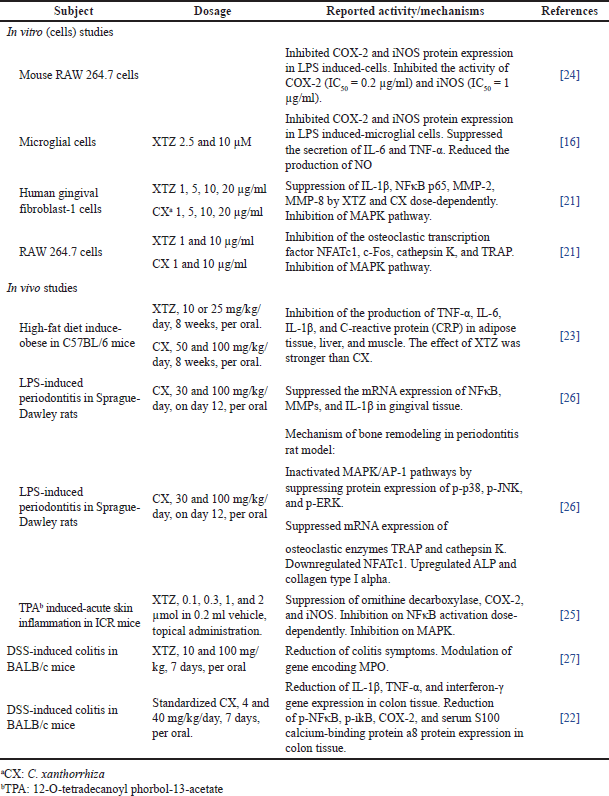 | Table 1. Regulation of inflammatory mediators by XTZ and C. xanthorrhiza extract. [Click here to view] |
XTZ can modulate the arachidonic signaling pathway. In the activated RAW 264.7 system with accumulated prostaglandin E2 (Pi2) and NO radicals, treatment with XTZ was able to attenuate NO radicals [24].
The ability of XTZ and C. xanthorrhiza to downregulate the NFκB pathway was also reported. XTZ treatment in periodontitis-induced rats was able to reduce the mRNA expression of NFκB [26]. In another study using DSS-induced colitis mice, C. xanthorrhiza was reported to reduce the protein expression of NFκB, a phosphorylated inhibitor of nuclear factor kappa B-α (p-iκB), and COX-2, indicating the downregulation of NFκ pathway [22]. The potential of XTZ and C. xanthorrhiza to treat colitis was also supported by their activity to downregulate 34 genes encoding myeloperoxidase (MPO), which is the enzyme participating in neutrophil infiltration in colitis [27]
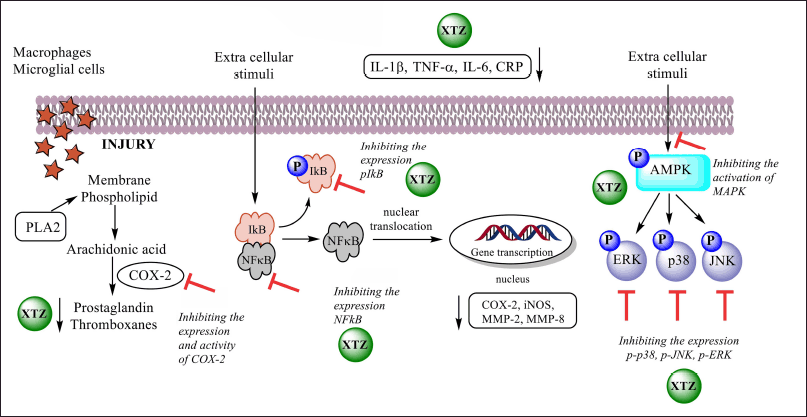 | Figure 3. Molecular pathways in inflammation modulated by XTZ. [Click here to view] |
XTZ and supercritical C. xanthorrhiza extract regulated the mitogen-activated protein kinase (MAPK) pathway in lipopolysaccharide (LPS)-treated HGF-1 cells, as seen by decreased phosphorylation of extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases (JNK), and p38 due to treatment with XTZ and C. xanthorrhiza [26]. It has been known that MAPK is involved in osteoclastogenesis, which causes bone resorption [28]. XTZ and C. xanthorrhiza may have benefits in the bone remodeling process in periodontitis. It has been known that continuous inflammation in periodontitis causes osteoclastogenesis, which leads to bone resorption. XTZ and C. xanthorrhiza inhibited the protein expression of the osteoclastic enzymes tartrate-resistant acid phosphatase (TRAP) and cathepsin K, which are involved in osteoclastogenesis, and downregulated the osteoclastic transcription factor nuclear factor of activated T-cells c1 (NFATc1) [26].
XTZ as an antimicrobial agent
Traditionally, C. xanthorrhiza has been used to treat gastrointestinal problems [29], which could be due to microbial infection. Studies have been conducted to investigate the antimicrobial activities of C. xanthorrhiza and XTZ against Gram-positive bacteria [30,31], Gram-negative bacteria [31], and fungi [32], as shown in Tables 2 and 3. Different methods were employed to evaluate the activities, such as susceptibility tests, adherence and biofilm formation test, viability test, and docking study.
In general, XTZ exhibited broad-spectrum antimicrobial activity compared to C. xanthorrhiza extracts. In particular, XTZ showed potent inhibition activity against Gram-positive bacteria; however, weak activities were observed against Gram-negative bacteria (Table 1). The different effects of XTZ on Gram-positive and Gram-negative bacteria could be due to the cell wall composition. The outer membrane peptidoglycan layers of the Gram-positive bacteria are considered more hydrophobic than the phospholipid structure of the Gram-negative bacteria [41,42]; therefore, they are likely more susceptible to plant-based phenolic compounds, including XTZ. XTZ has also shown antifungal activity (Tables 1 and 2), which may be ascribed to the hydrophobic nature of the fungi cell wall [43], which is likely more permeable to XTZ. By targeting cell walls, XTZ may disrupt the integrity of the microbial cell wall leading to membrane lysis [44].
Notably, different Streptococcus species implicated in dental caries are particularly susceptible to XTZ. High inhibition activity was observed against A. viscosus and P. gingivalis, which are responsible for periodontitis. There were reports on the antibiofilm adherence and formation of periodontal microbes, including S. mutans and C. albicans [45].
Synergistic effects were observed for the combination of XTZ with other compounds. A study by Kim et al. [33] reported that a combination of XTZ and food-grade materials, such as polymyxin B nonapeptide, resulted in higher susceptibility against E. coli, S. enterica serovar Typhimurium, and V. cholerae. These bacteria are associated with food-borne bacterial infections.
A combination of XTZ and synthetic drugs can potentiate the antifungal activity of the drugs. The synergistic effect was reported for combinations of XTZ and ketoconazole or amphotericin B [34]. Combinations of XTZ (1/2 MIC) and ketoconazole (1/2 MIC) or XTZ (1/2 MIC) and amphotericin B (1/2 MIC) reduced the number of viable cells by more than 3 log units for all Candida species [34]. Recently, a combination of XTZ and experimental ?uoride varnish (EFV) was evidenced to enhance XTZ inhibition activity against S. mutans [46]. XTZ (1 or 10 μM) and EFV (1:1) gave a more effective inhibition than EFV only.
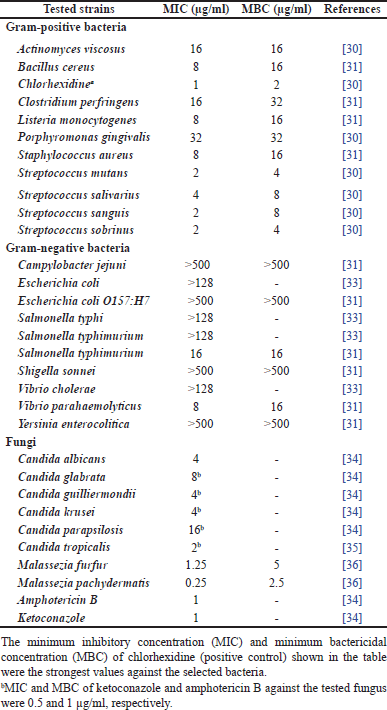 | Table 2. Antimicrobial activities of xantorrhizol (MIC and MBC). [Click here to view] |
The antibacterial activity of XTZ can be attributed to its anti-inflammatory effect. It was reported that inflammatory factors such as NF-κB p65 and IL-1β were overexpressed in LPS-treated human gingival fibroblast-1 cells. However, treatment with XTZ significantly downregulated the expression of NF-κB p65 and IL-1β, suggesting XTZ’s ability to inhibit MAPK/AP-1 signaling pathway [21]. Further, a docking study showed the mechanism of inhibition of XTZ against E. coli. XTZ targeted the FabI enzyme, which is an enoyl-ACP reductase. FabI is responsible for the catalyzes the last step in the biosynthesis of fatty acids. Binding of XTZ to FabI:NAD+ inactivated the enzyme in a different manner from triclosan [47].
Ample studies have reported the antimicrobial activity of XTZ and C. xanthorrhiza, thus supporting its traditional claim for the treatment of microbial infection-related diseases. However, bioavailability studies are required to potentially utilize XTZ as alternative antibiotics.
XTZ as a hepatoprotective agent
Traditionally, C. xanthorrhiza has been used to cure liver complaints such as hepatitis and jaundice. The hepatoprotective effect of C. xanthorrhiza has been studied using different hepatotoxic rats or mice models induced by CCl4 [48], ethanol [49], aspartame [50], and paracetamol [51]. Consumption of C. xanthorrhiza before liver injury improved biochemical parameters of the liver, including the decreased level of serum liver enzymes [alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), serum glutamic oxaloacetic transaminase (SGOT), and serum glutamic pyruvic transaminase (SGPT)], which contribute to the hepatoprotective activity of C. xanthorrhiza. In addition, administration of C. xanthorrhiza increased liver antioxidant enzymes (SOD, CAT, gluthatione peroxidase (GPx), decreased lipid peroxidation as seen by lowered MDA level, and improved recovery of hepatic tissue.
The hepatoprotective activity of XTZ isolated from C. xanthorrhiza was reported by Kim et al. [52] using cisplatin-induced hepatotoxicity. Cisplatin is an anticancer drug that, upon high-dose administration, caused hepatotoxicity. Pretreatment with XTZ (200 mg/g BW, p.o.) for four days before cisplatin induction (45 mg/kg BW, i.p.) decreased serum SGOT, SGPT, and γ-GT levels compared to the control group. It was noted that curcumin at the same concentration (200 mg/g BW) led to less effect than that of XTZ, even at half the concentration (100 mg/g BW). The hepatoprotective effect of XTZ is attributed to its ability to regulate gene transcription involved in hepatotoxicity. Some hepatotoxins are known to increase the activation of NF-κB and activator protein-1 (AP-1), which involve in the transcriptions of inflammatory genes in hepatotoxicity [53]. Cisplatin induction increased the DNA-binding activity in NF-κB and AP-1; however, this effect was modulated by XTZ. Pretreatment with XTZ suppressed the DNA-binding activity in NF-κB, subsequently suppressing the mRNA expression of NF-κB-related genes, COX-2 and iNOS [52]. This study identified that cisplatin induction increased the expression of S100 calcium-binding protein A9 (S100A9) mRNA and antigenic determinant for rec-A protein mRNA. In addition, cisplatin decreased the level of expression of caseinolytic protease X (ClpX) mRNA and ceruloplasmin (CP) mRNA. However, pretreatment with XTZ reversed the alteration in these gene expressions [52].
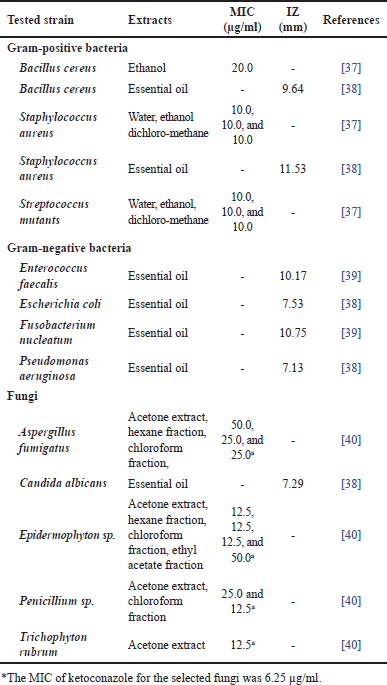 | Table 3. Antimicrobial activities of C. xanthorrhiza. [Click here to view] |
In addition to this mechanism, the preventive effect of XTZ is associated with its modulation of MAPK signaling. Cisplatin-induced the phosphorylation of JNK 1/2 and the ERK 1/2. However, pretreatment with XTZ highly suppressed the phosphorylation of JNK 1/2 and ERK 1/2 dose-dependently. In addition, XTZ pretreatment also partially regulated the transcription subunits c-fos and p50, which play a role in the activation of AP-1 [54].
The ability of XTZ to regulate gene expression was supported by findings using a computational target fishing study. XTZ targeted multiple pathways connected with 40 genes, four of which (RXRA, RBP4, HSD11B1, and AKR1C1) were greatly upregulated. These genes were mainly involved in the steroid metabolic process. By gene ontology (GO) and KEGG pathway analysis, the method identified thirteen genes linked with xenobiotics by cytochrome P450 which were highly expressed [55].
In addition to hepatoprotective activity, C. xanthorrhiza and XTZ were studied for their inhibition activity on human liver glucuronidation [56]. Liver glucuronidation is responsible for the excretion of xenobiotics and endobiotics, including drugs, from the body. XTZ showed weak glucuronidation inhibition activity compared to curcumin (IC50 of 117.55 and 11.88 μg/ml, respectively). However, the inhibition activity was stronger than the aqueous extract of C. xanthorrhiza (IC50 of 271.10 μg/ml). These findings reflect the possible interaction of herbal compounds with drugs that undergo the same glucuronidation, thus may disrupt their efficacy. Recently, curcumin (500 and 1,000 mg/day) was proven to be efficacious in improving various hepatic parameters in nonfatty liver disease in clinical trials [57,58]. The above results regarding XTZ have confirmed the hepatoprotective activity of XTZ. However, more studies are required to evaluate its application in clinical settings.
XTZ as an antidiabetic and antihyperlipidemic agent
Growing evidence has reported that glucose and lipid metabolism disorders are closely related to the onset and progression of diabetes, obesity, cardiovascular disease, and cancer [59,60]. Therefore, alternative therapies to combat glucose and lipid metabolism disorders would have significant medical impacts. Multiple studies have demonstrated the potential antidiabetic activity of C. xanthorrhiza and XTZ, in which treatments with C. xanthorrhiza and XTZ improve glucose and lipid metabolisms. Treatment with C. xanthorrhiza extract (5%, 2 weeks, p.o) in streptozotocin-induced mice decreased serum glucose and TG levels [61]. Similarly, using obesity-induced hyperglycemia, C. xanthorrhiza extract (50 and 100 mg/kg, 8 weeks, co-administered with high-fat diet, p.o), led to a significant decrease in post prandial serum glucose by 28.5% and 31.2% [23].
Treatment with XTZ isolated from C. xanthorrhiza (10 or 25 mg/kg BW) had a similar effect on postprandial glucose levels (21.8% and 33%) and the results were comparable with those of metformin treatment (100 mg/kg BW) [23]. Further, it was observed that serum FFA and TG levels, epididymal fat pad, and adipocyte size were also decreased by treatment with C. xanthorrhiza or XTZ [23]. It should be noted that inflammatory cytokines TNF-α, IL-6, IL-1β, and CRP were found overexpressed in the liver, muscle, and adipose tissues of the high fat-induced diabetic mouse. It has been reported that enlarged adipocytes produce proinflammatory cytokines and chemokines [62]. Importantly, treatment with C. xanthorrhiza or XZ in obese mice decreased the expression of inflammatory cytokines TNF-α, IL-6, IL-1β, and CRP of the liver, muscle, and adipose tissues compared to the control group. Fat accumulation induced an inflammatory response in those organs, which in turn interfered with insulin signaling and β-cells dysfunction [63].
The antidiabetic activity of XTZ and C. xanthorrhiza may also be accounted for their inhibition activity on α-glucosidase. This enzyme catalyzes the hydrolysis of dietary polysaccharides into glucose in the small intestine; thus, inhibition of α-glucosidase reduces the absorption of glucose into the blood circulation. Ethanol and aqueous ethanol extracts of C. xanthorrhiza inhibited α-glucosidase dose-dependently, with IC50 of 28.4 and 36.6 μg/ml [13]. However, very weak inhibition activity was observed using ethyl acetate fractions of the methanol extract (IC50 339.05–455.01 μg/ml) [64].
The lipid-lowering effect previously mentioned [23] is likely associated with pancreatic lipase inhibition activity. Batubara et al. [14] reported that ethyl acetate fraction of C. xanthorrhiza inhibited pancreatic lipase in vitro with activity comparable to that of catechin (at 500 μg/ml each gave 80.5% and 81.4% inhibition). However, methanol and hexane fractions and XTZ gave weak inhibition activity (5.90%, 9.10%, and 36.09%, respectively, at 500 μg/ml) [14].
The lipid-lowering effect of XTZ may also be contributed by its effect in reducing cholesterol uptake in the intestine. In a study using HT29 colon cells, XTZ treatment (15 μg/ml) in the cultured cells led to cholesterol uptake reduction by 27%, which is not significantly different from the control [65]. The same authors reported that XTZ treatment reduced the growth of 3TL1 pre-adipocytes and adipocytes. It is known that a reduced number of adipocytes may reduce fat accumulation. It was observed that at 35 μg/ml, XTZ inhibited 50% of 3TL1 adipocytes.
The findings above exhibited that C. xanthorrhiza and XTZ are potential sources of antihyperglycemia agents. More studies are required to further understand its mechanism of action. On the other hand, curcumin has been reported for its antidiabetic activity in pre- and clinical settings [66]. The hypoglycemic effect of a combination of XTZ and curcumin is not yet reported. Studies regarding the potential synergistic effect of XTZ and curcumin could be beneficial for future drug development.
XTZ as a neuroprotective agent
Neurotoxicity has been implicated in different neurodegenerative disorders, including Parkinson’s disease, Alzheimer’s disease, and hyperactivity disorder. XTZ has been studied for its protective effect against oxidative and inflammatory toxicity. Its ability to modulate the expression of inflammatory proteins influences neuroactivities. Lim et al. [67] found that activation of microglial cells by LPS induction increased NO and upregulated iNOS and COX-2. These effects were reversed in the XTZ-treated cells. XTZ downregulated the expression of iNOS and COX-2 dose-dependently and decreased NO production, suggesting that nitrite suppression is due to the downregulation of iNOS.
In addition, XTZ was found to decrease proin?ammatory cytokines. Treatment with LPS in microglial cells increased proinflammatory cytokines (IL-6, TNF-α) and nitrite. However, treatment with XTZ suppressed the overexpression of proinflammatory cytokines in a dose-dependent manner [67]. This suggests that the neuroprotective activity of XTZ is associated with its inhibition of neuroinflammation.
Lim and Han [17] investigated the protective effect of XTZ against neurotoxicity in human neuroblastoma SK-N-SH cells. Alzheimer’s disease is typified by extensive plaque deposition of Aβ peptides. Increased deposit of Aβ induces ROS generation, including HNE. The oxidative stress by increased HNE decreases the activity of neprilysin, the main protein that cleaves Aβ. Therefore, drugs that suppress the modification of neprilysin could be effective in treating AD. XTZ treatment in SK-N-SH cells prevented modification of neprilysin due to HNE induction. XTZ (10 μM) reduced HNE level by 59% on neprilysin protein. Notably, the inactivation of neprilysin was prevented with treatment with XTZ. This effect was observed by the reduction of HNE positive signal in the Aβ42 induced cells treated with XTZ (10 μM). Further, treatment with XTZ (1 and 10 μM) was able to cleave Aβ42 by 46% and 63%, respectively, indicating that XTZ is able to enhance neprilysin activation.
The neuroprotective activity of XTZ has been studied using murine HT22 hippocampal neuron cells. Pretreatment with XTZ increased cell viability compared to control (glutamate-induced neurotoxicity). In addition, treatment with XTZ suppressed the amount of ROS in glutamate-induced HT22 cells. These findings suggest that the neuroprotective activity of XTZ is due to its antioxidant effect. This effect is similar to that observed for curcumin. In addition, XTZ reduced H2O2 lipid peroxidation dose-dependently in the rat brain homogenate (completely inhibited lipid peroxidation at 10 μM).
The involvement of histamine in the central nervous system has been well evidenced in the pathophysiology of different neurological disorders, such as attention deficit and hyperactivity disorders [68]. Thus, drugs that target histamine N-methyltransferase are key for treating neurological-related disorders. By using in silico approach, 26 secondary metabolites of C. xanthorrhiza were screened, and 7 of them were predicted to be developed as HNMT inhibitors [69].
Taken together, the results above demonstrated the protective role of XTZ against neurotoxicity. Its protective role is associated with the ability of XTZ to inhibit monoamine oxidase (MAO) and acetylcholine esterase. Both enzymes are implicated in neurodegenerative illnesses. MAO, a flavoprotein, catalyzes the oxidative deamination of various neurotransmitters (such as dopamine and serotonin) and exogenous amines, thus being involved in the regulation of neurotransmitters [70]. Acetylcholinesterase catalyzes the hydrolysin of the acetylcholine neurotransmitter. In the case of curcumin, it was reported to inhibit MAO activity competitively [71] and was also able to decrease the expression of acetylcholinesterase in rotenone-induced mice [72].
XTZ as a nephroprotective agent
XTZ can be a promising nephroprotective agent. Notably, XTZ has exhibited a stronger nephroprotective effect than curcumin [73]. XTZ attenuated cisplatin-induced DNA binding activity of the transcription factors NF-κB. However, XTZ did not show an effect on AP-1, suggesting that the protective effect of XTZ is due to its inhibition of the DNA binding activity of NF-κB.
In addition, XTZ was shown to ameliorate cisplatin-induced nephrotoxicity. Cisplatin is a potent chemotherapy drug in the treatment of cancer. However, its use is limited by its toxicity against normal tissues and organs, including the kidney. Many cisplatin-receiving cancer patients present signs of nephrotoxicity 10 days after treatment [74]. Cisplatin administration can increase blood urea nitrogen and creatinine. However, XTZ pretreatment before cisplatin administration decreased the levels of blood urea nitrogen and creatinine. This study has demonstrated that XTZ can be a promising approach to ameliorating cisplatin-induced nephrotoxicity. However, further studies are needed to investigate whether XTZ pretreatment interferes with the cisplatin metabolic pathway, thus may reduce cisplatin’s efficacy.
XTZ as a skin protective agent
Traditionally, C. xanthorrhiza has been used to treat skin inflammation. Studies have reported the protective effect of C. xanthorrhiza and XTZ against skin-related diseases, in particular microbial infection, and its protective effect against damage due to UV exposure. Previously, the dried flower bract of C. xanthorrhiza has been studied in vitro for its skincare potential. Different extracts (hexane, ethyl acetate, and methanol) and essential oil showed weak inhibition activity against Propionibacterium acnes [14] and weak anti-tyrosinase activities [14].
Excessive and prolonged exposure to UV radiation disrupts dermal antioxidant function. Quantitatively, SOD, CAT, and GSH decrease, producing unregulated ROS [75]. Further, UV radiation upregulates the expression of MMP-1, which is responsible for the degradation of collagen and dermal connective tissue [76]. UV-protective agents are therefore needed to minimize the photodamage in dermal structure and skin aging. The skin protective activity of XTZ was studied in human skin fibroblasts (HaCaT). Following irradiation of cultured fibroblast with UV (20 mJ/cm2), the MMP-1 was upregulated, whereas type 1 procollagen expression was downregulated. However, treatment with XTZ suppressed the expression of MMP-1 and increased the expression of type 1 procollagen in a dose-dependent manner. At 0.1 μM, XTZ nearly abrogated the MMP-1 expression (reduction by 92%) and increased pro-collagen expression by 86%. These effects were more effective than those observed in the group treated with EGCG (0.1 μM, reduced MMP-1 expression by 72% and increased pro-collagen expression by 65%). It is worth noting that methanol extract of C. xanthorrhiza downregulated MMP-1 and upregulated type 1 procollagen in a dose-dependent manner. These findings clearly demonstrated the anti-photoaging activity of XTZ and C. xanthorrhiza. In another study, curcumin was also shown to have a photoprotective effect against UVB radiation by the upregulation of nuclear factor erythroid factor-2, which regulates the expression of antioxidant enzymes, including SOD and CAT [77]. In addition to XTZ, 13-hydroxyxanthorrhizol (HXTZ), isolated from the methanolic extract of C. xanthorrhiza, was also found to have protective activity against UVB irradiation [78]. A study in keratinocyte cells showed that treatment with HXTZ (5 μM) attenuated the expression of MMP-1 (by 6.6 folds), indicating that HXTZ inhibited the production of MMP-1. The photo-protective effect of XTZ shows evidence of its potential as an anti-aging agent. Further studies are necessary to understand other mechanisms that contribute to skin antiaging activities, such as the possibility of XTZ to prevent water loss in the trans-epidermal through its modulation of aquaporin expression [79].
Toxicity studies
To date, studies are still limited regarding the toxicity of XTZ and C. xanthorrhiza. Yamazaki et al. [80] found that in pentobarbital-induced mice, XTZ administration at 500 mg/kg did not cause a significant effect on behavior. The study did not report mortality for this dosage. Devaraj et al. [81] reported the safety of C. xanthorrhiza ethanol extract in an acute oral toxicity test in mice as per OECD guidelines no 423. No mortality was reported after administering a single oral dose of 300, 2,000, and 5,000 mg/kg to mice [81]. Toxicity signs were not observed in the skin, fur, and eyes of the mice within the 14 days of the study. Behavior changes were not noticeable in the test mice. Listyawati et al. [82] carried out a chronic toxicity test of C. xanthorrhiza ethanol extract in mice treated with 150 mg/kg daily for 90 days. No mortality was reported, and the treatment did not cause changes in mice behavior and external morphology. The treatment did not cause significant changes in hematological and spermatogenic parameters. The above in vivo studies show that XTZ and C. xanthorrhiza are nontoxic to mice. However, the mutagenicity, carcinogenicity, and genotoxicity of C. xanthorrhiza and its active compounds, particularly XTZ, need to be studied to allow further application.
Future research prospects
Curcuma xanthorrhiza has long found its use in the traditional medicine of Indonesia. It is noted that C. xanthorrhiza is an essential part of jamu (traditional formulation). The present review may support C. xanthorrhiza traditional use for treating liver, gastrointestinal, and skin complaints, as well as infection, metabolic syndrome, and cardiovascular problems. Traditionally, C. xanthorrhiza has been used to eliminate fatigue and maintain body wellness [2,83,84]. Despite this traditional claim, scientific reports regarding these claims are insufficient. Therefore, C. xanthorrhiza and XTZ’s role in energy production should be further investigated. Fatigue is a complex condition related to various mechanisms, including the endocrine system, central and peripheral nervous system, metabolic activity, immune system, and oxidative stress [85]. Existing evidence suggests that C. xanthorrhiza and XTZ may have good potential as antioxidant agents, warranted by observation in the suppression and inhibition of ROS using cell lines [67] and inhibition of lipid peroxidation in human LDL [15].
During intense exercise, ROS production increases in the mitochondria of the muscle cells. ROS damages the skeletal muscles and liver mitochondrial membrane caused by lipid peroxidation. In addition, potential antifatigue activity by XTZ may also be accounted for by its anti-inflammatory activity, including XTZ’s impact on suppressing proinflammatory factors (IL-1β) and inflammatory mediators (Fig. 2). Previously, curcumin has been demonstrated to have an antifatigue effect in animal models. Treatment with curcumin led to longer swimming time [86–88], increased liver antioxidant profile (SOD) [86], and decreased biochemical parameters related to fatigue, such as blood urea nitrogen, AST, ALT, and creatinine kinase [88]. These effects could be attributed to curcumin regulation in mitochondria biogenesis in the skeletal muscle, as seen by activation of AMP-activated protein kinase pathway, increased expression of sirtuin-1, peroxisome proliferator-activated receptor-gamma coactivator-1α, ox-phos subunits, and mtDNA copy number [87]. On the other hand, studies regarding XTZ’s effect on mitochondria biogenesis and its possible role as a bioenergetic stimulant are scarce. Therefore, future studies may be directed at the investigation of the antifatigue activity of C. xanthorrhiza and XTZ.
CONCLUSION
Xanthorrihizol, a bisabolene sesquiterpenoid, is abundant in the rhizomes of C. xanthorrhiza. The current review has demonstrated that XTZ is a promising compound to develop antimicrobial, antidiabetic, and anti-hyperlipidemia, in addition to hepato-, nephron-, neuro-, and skin-protective agents. Although its potential is not fully investigated, several pharmacological studies have shown that XTZ interacts with multiple biological targets exhibited in different host cells in both in vitro and in vivo, which involves inflammation and oxidative stress in different pathologies. This compound is worth further studying, considering various traditional uses of C. xanthoorhiza, the main source of XTZ. Limited toxicity studies of XTZ necessitate further investigations to allow its applications in clinical settings. It is important to perform clinical studies to understand the pharmacokinetics of XTZ, as the application of XTZ in the body alters molecular, cellular, tissue, or organ levels.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data generated and analyzed are included within this report.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hirschhorn HH. Botanical remedies of the former Dutch East Indies (Indonesia). Part II: dicotyledones up to and including leguminosae. J Ethnopharmacol. 1983;8(1):65–96. doi: https://doi.org/10.1016/0378-8741(83)90090-9
2. Sampurno OD, Purba MP, Sintawat K, Walujo EB, Hernawan BH, Purwanto D, et al. Ramuan obat tradisional Indonesia serat centhnini, buku jampi, kitab Tibb. Jakarta, Indonesia: Badan Pengawasan Obat dan Makanan Republik Indonesia, Deputi Bidang Pengawasan Obat Tradisional, Kosmetik dan Produk Komplemen Direktorat Obat Asli Indonesia; 2016.
3. Klau ME, Rohaeti E, Rafi M, Artika IM, Ambarsari L, Nurcholis W. Metabolite profiling of Curcuma xanthorriza varieties grown in different regions using UHPLC-Q-Orbitrap-HRMS and chemometrics analysis. Biointerface Res Appl Chem. 2023;13(1):1–13.
4. Mary HPA, Susheela GK, Jayasree S, Nizzy AM, Rajagopal B, Jeeva S. Phytochemical characterization and antimicrobial activity of Curcuma xanthorrhiza Roxb. Asian Pac J Trop Biomed. 2012;2(2, Supplement):S637–40. doi: https://doi.org/10.1016/S2221-1691(12)60288-3
5. Lim J, Nguyen TTH, Pal K, Gil Kang C, Park C, Kim SW, et al. Phytochemical properties and functional characteristics of wild turmeric (Curcuma aromatica) fermented with Rhizopus oligosporus. Food Chem X. 2022;13:100198. doi: https://doi.org/10.1016/j.fochx.2021.100198
6. Duong L, Mentreddy SR, Satyal R, Satyal P, Setzer WN. Essential oil chemotypes of four Vietnamese Curcuma species cultivated in North Alabama. Horticulturae 2022;8(5):360–77.
7. Jarikasem S, Thubthimthed S, Chawananoraseth K, Suntorntanasat T, editors. Essential oils from three Curcuma species were collected in Thailand. III WOCMAP Congress on Medicinal and Aromatic Plants-Volume 3: Perspectives in Natural Product Chemistry, Chiang Mai, Thailand, vol. 677; 2003.
8. Srivastava AK, Srivastava SK, Syamsundar KV. Volatile composition of Curcuma angustifolia Roxb. rhizome from central and southern India. Flavour Fragrance J. 2006;21(3):423–6. doi: https://doi.org/10.1002/ffj.1680
9. Simamora A, Timotius KH, Yerer MB, Setiawan H, Mun’im A. Xanthorrhizol, a potential anticancer agent, from Curcuma xanthorrhiza Roxb. Phytomedicine. 2022;105:154359. doi: https://doi.org/10.1016/j.phymed.2022.154359
10. Chatterjee S. Chapter two—oxidative stress, inflammation, and disease. In: Dziubla T, Butterfield DA, editors. Oxidative stress and biomaterials. New York, NY: Academic Press; 2016. pp 35–58.
11. Akbarian A, Michiels J, Golian A, Buyse J, Wang Y, De Smet S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult Sci. 2014;93(8):1930–41. doi: https://doi.org/10.3382/ps.2014-03896
12. Awin T, Buzgaia N, Abd Ghafar SZ, Mediani A, Mohd Faudzi SM, Maulidiani M, et al. Identification of nitric oxide inhibitory compounds from the rhizome of Curcuma xanthorrhiza. Food Biosci. 2019;29:126–34. doi: https://doi.org/10.1016/j.fbio.2019.04.009
13. Awin T, Mediani A, Maulidiani, Shaari K, Faudzi SMM, Sukari MAH, et al. Phytochemical profiles and biological activities of Curcuma species subjected to different drying methods and solvent systems: NMR-based metabolomics approach. Ind Crops Prod. 2016;94:342–52. doi: https://doi.org/10.1016/j.indcrop.2016.08.020
14. Jantan I, Saputri FC, Qaisar MN, Buang F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evid Based Complement Alternat Med. 2012;2012:438356. doi: https://doi.org/10.1155/2012/438356
15. Batubara I, Julita I, Darusman LK, Muddathir AM, Mitsunaga T. Flower bracts of temulawak (Curcuma xanthorrhiza) for skin care: anti-acne and whitening agents. Proc Chem. 2015;14:216–24. doi: https://doi.org/10.1016/j.proche.2015.03.031
16. Lim CS, Jin D-Q, Mok H, Oh SJ, Lee JU, Hwang JK, et al. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005;82(6):831–8. doi: https://doi.org/10.1002/jnr.20692
17. Lim Chol S, Han J-S. The antioxidant xanthorrhizol prevents amyloid-β-induced oxidative modification and inactivation of neprilysin. Biosci Rep. 2018;38(1). doi: https://doi.org/10.1042/bsr20171611
18. Ichikawa K, Sasada R, Chiba K, Gotoh HJA. Effect of side chain functional groups on the DPPH radical scavenging activity of bisabolane-type phenols. Antioxidants. 2019;8(3):65. doi: https://doi.org/10.3390/antiox8030065
19. Tasneem S, Liu B, Li B, Choudhary MI, Wang W. Molecular pharmacology of inflammation: medicinal plants as anti-inflammatory agents. Pharmacol Res. 2019;139:126–40. doi: https://doi.org/10.1016/j.phrs.2018.11.001
20. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563–82. doi: https://doi.org/10.1016/j.bbamcr.2014.05.014
21. Kim S, Kook KE, Kim C, Hwang J-K. Inhibitory effects of Curcuma xanthorrhiza supercritical extract and xanthorrhizol on LPS-induced inflammation in HGF-1 cells and RANKL-induced osteoclastogenesis in RAW264. 7 cells. J Microbiol Biotechnol Adv. 2018;28(8):1270–81.
22. Young Cho J, Yeon Kim H, Me Kim H, Na Song H, Hong E, Hwang J-K, et al. Standardized ethanolic extract of the rhizome of Curcuma xanthorrhiza prevents murine ulcerative colitis by regulation of inflammation. J Funct Foods. 2017;30:282–9. doi: https://doi.org/10.1016/j.jff.2017.01.020
23. Kim MB, Kim C, Song Y, Hwang JK. Antihyperglycemic and anti-inflammatory effects of standardized Curcuma xanthorrhiza Roxb. extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evid Based Complement Altern Med. 2014;2014:205915. doi: https://doi.org/10.1155/2014/205915
24. Lee SK, Hong C-H, Huh S-K, Kim S-S, Oh O-J, Min H-Y, et al. Suppressive effect of natural sesquiterpenoids on inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) activity in mouse macrophage cells. J Environ Pathol Toxicol Oncol. 2002;21(2):141–8.
25. Chung WY, Park JH, Kim MJ, Kim HO, Hwang JK, Lee SK, et al. Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-κB. Carcinogenesis. 2007;28(6):1224–31. doi: https://doi.org/10.1093/carcin/bgm005
26. Kook KE, Kim C, Kang W, Hwang J-K. Inhibitory effect of standardized Curcuma xanthorrhiza supercritical extract on LPS-induced periodontitis in rats. J Microbiol Biotechnol. 2018;28(10):1614–25. doi: https://doi.org/10.4014/jmb.1808.08052
27. Cho JY, Hwang J-K, Chun HS. Xanthorrhizol attenuates dextran sulfate sodium-induced colitis via the modulation of the expression of inflammatory genes in mice. Life Sci. 2011;88(19):864–70. doi: https://doi.org/10.1016/j.lfs.2011.03.007
28. He X, Andersson G, Lindgren U, Li Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun. 2010;401(3):356–62. doi: https://doi.org/10.1016/j.bbrc.2010.09.053
29. Dewi M, Aries M, Dwiriani CM, Januwati N. Knowledge on health benefit of temulawak (Curcuma xanthorrhiza) and the clinical trial of Its effect on humoral immune system in obese adults (Pengetahuan tentang manfaat kesehatan temulawak (Curcuma xanthorrhiza.) serta uji klinis pengaruhnya pada sistem imun humoral pada dewasa obes). JIPI. 2012;17(3):166–71.
30. Hwang JK, Shim JS, Pyun YR. Antibacterial activity of xanthorrhizol from Curcuma xanthorrhiza against oral pathogens. Fitoterapia. 2000;71(3):321–3. doi: https://doi.org/10.1016/S0367-326X(99)00170-7
31. Lee LY, Shim J-S, Rukayadi Y, Hwang J-K. Antibacterial activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. against foodborne pathogens. J Food Prot. 2008;71(9):1926–30.
32. Rukayadi Y, Hwang J-K. In vitro antimycotic activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. against opportunistic filamentous fungi. Phytother Res. 2007;21(5):434–8. doi: https://doi.org/10.1002/ptr.2092
33. Kim MS, Kim H-R, Kim H, Choi S-K, Kim C-H, Hwang J-K, et al. Expansion of antibacterial spectrum of xanthorrhizol against Gram-negatives in combination with PMBN and food-grade antimicrobials. J Microbiol. 2019;57(5):405–12.
34. Rukayadi Y, Lee K, Lee MS, Yong D, Hwang JK. Synergistic anticandidal activity of xanthorrhizol in combination with ketoconazole or amphotericin B. FEMS Yeast Res. 2009;9(8):1302–11. doi: https://doi.org/10.1111/j.1567-1364.2009.00548.x
35. Rukayadi Y, Lee K, Lee M-S, Yong D, Hwang J-KJFyr. Synergistic anticandidal activity of xanthorrhizol in combination with ketoconazole or amphotericin B. FEMS Yeast Res. 2009;9(8):1302–11.
36. Rukayadi Y, Hwang J-K. In vitro anti-Malassezia activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. Lett Appl Microbiol. 2007;44(2):126–30.
37. Mangunwardoyo W, Usia T. Antimicrobial and identification of active compound Curcuma xanthorrhiza Roxb. Int J Basic Appl Sci. 2012;12(1):69–78.
38. Akarchariya N, Sirilun S, Julsrigival J, Chansakaowa S. Chemical profiling and antimicrobial activity of essential oil from Curcuma aeruginosa Roxb., Curcuma glans K. Larsen & J. Mood and Curcuma cf. xanthorrhiza Roxb. collected in Thailand. Asian Pac J Trop Biomed. 2017;7(10):881–5. doi: https://doi.org/10.1016/j.apjtb.2017.09.009
39. Prijatmoko D, Syafira NL, Lestari PE. Antibacterial activity of essential oil extracts from Curcuma xanthorrhiza roxb. rhizomes against bacteria causing pulp necrosis. J Dentomaxillofac Sci. 2018;3(3):144–8.
40. Diastuti H, Asnani A, Chasani M, editors. Antifungal activity of Curcuma xanthorrhiza and Curcuma soloensis extracts and fractions. IOP Conf Ser Mater Sci Eng. 2019;509:012047.
41. Bouarab-Chibane L, Forquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, et al. Antibacterial properties of polyphenols: characterization and QSAR (quantitative structure–activity relationship) models. Front Microbiol. 2019;10:829. doi: https://doi.org/10.3389/fmicb.2019.00829
42. Inouye S, Yamaguchi H, Takizawa T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J Infect Chemother. 2001;7(4):251–4. doi: https://doi.org/10.1007/s101560170022
43. Hazen KC, Glee PM. Cell surface hydrophobicity and medically important fungi. Curr Topics Med Mycol. 1995;6:1–31.
44. Zheng D, Huang C, Huang H, Zhao Y, Khan MRU, Zhao H, et al. Antibacterial mechanism of curcumin: a review. Chem Biodivers. 2020;17(8):e2000171. doi: https://doi.org/10.1002/cbdv.202000171
45. Rukayadi Y, Hwang J-K. In vitro activity of xanthorrhizol against Streptococcus mutans biofilms. Lett Appl Microbiol. 2006;42(4):400–4.
46. Son J-L, Kim A-J, Oh S, Bae J-M. Inhibitory effects on Streptococcus mutans of antibacterial agents mixed with experimental fluoride varnish. Dent Mater J. 2020;39(4):690–5.
47. Yogiara, Mordukhova EA, Kim D, Kim W-G, Hwang J-K, Pan J-G. The food-grade antimicrobial xanthorrhizol targets the enoyl-ACP reductase (FabI) in Escherichia coli. Bioorg Med Chem Lett. 2020;30(24):127651. doi: https://doi.org/10.1016/j.bmcl.2020.127651
48. Devaraj S, Ismail S, Ramanathan S, Yam MF. Investigation of antioxidant and hepatoprotective activity of standardized Curcuma xanthorrhiza rhizome in carbon tetrachloride-induced hepatic damaged rats. Sci World J. 2014;2014:353128. doi: https://dx.doi.org/10.3390%2Fmolecules15042925
49. Devaraj S, Ismail S, Ramanathan S, Marimuthu S, Fei YM. Evaluation of the hepatoprotective activity of standardized ethanolic extract of Curcuma xanthorrhiza Roxb. J Med Plant Res. 2010;4(23):2512–7. doi: https://dx.doi.org/10.3390%2Fmolecules15042925
50. Suryani D, Lubis HML. Comparison between Ocimum sanctum hepatoprotector extract and Curcuma Xanthorrhiza on the histological structure of aspartame-induced Wistar rats. Bp Int Res Exact Sci. 2019;1(4):45–52. doi: https://doi.org/10.33258/birex.v1i4.476
51. Pramono S, Arifah FH, Pribadi FH, Nugroho AE. Hepatoprotective activity of Curcuma xanthorrhiza Roxb. paracetamol-induced liver damage in rats and correlation with their chemical compounds. TJPS. 2018;42(4):188–95.
52. Kim SH, Hong KO, Chung WY, Hwang JK, Park KK. Abrogation of cisplatin-induced hepatotoxicity in mice by xanthorrhizol is related to its effect on the regulation of gene transcription. Toxicol Appl Pharmacol. 2004;196(3):346–55. doi: https://doi.org/10.1016/j.taap.2004.01.002
53. Blazka ME, Bruccoleri A, Simeonova PP, Germolec DR, Pennypacker KR, Luster MI. Acetaminophen-induced hepatotoxicity is associated with early changes in AP-1 DNA binding activity. Res Commun Mol Pathol Pharmacol. 1996;92(3):259–73.
54. Hong KO, Hwang JK, Park KK, Kim SH. Phosphorylation of c-Jun N-terminal Kinases (JNKs) is involved in the preventive effect of xanthorrhizol on cisplatin-induced hepatotoxicity. Arch Toxicol. 2005;79(4):231–6. doi: https://doi.org/10.1007/s00204-004-0623-7
55. Shahid M, Azfaralariff A, Law D, Najm AA, Sanusi SA, Lim SJ, et al. Comprehensive computational target fishing approach to identify xanthorrhizol putative targets. Sci Rep. 2021;11(1):1594. doi: https://doi.org/10.1038/s41598-021-81026-9
56. Husni Z, Ismail S, Zulkiffli MH, Afandi A, Haron M. In vitro inhibitory effects of Andrographis paniculata, Gynura procumbens, Ficus deltoidea, and Curcuma xanthorrhiza extracts and constituents on human liver glucuronidation activity. Pharmacogn Mag. 2017;13(Suppl 2):S236–43. doi: https://doi.org/10.4103/pm.pm_299_16
57. Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, et al. Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res. 2016;30(9):1540–8. doi: https://doi.org/10.1002/ptr.5659
58. Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res. 2017;67(4):244–51. doi: https://doi.org/10.1055/s-0043-100019
59. Bays HE, Taub PR, Epstein E, Michos ED, Ferraro RA, Bailey AL, et al. Ten things to know about ten cardiovascular disease risk factors. Am J Prev Cardiol. 2021;5:100149. doi: https://doi.org/10.1016/j.ajpc.2021.100149
60. La Sala L, Pontiroli AE. Prevention of diabetes and cardiovascular disease in obesity. Int J Mol Sci. 2020;21(21):8178. doi: https://doi.org/10.3390/ijms21218178
61. Yasni S, Imaizumi K, Sugano M. Effects of an Indonesian medicinal plant, Curcuma xanthorrhiza Roxb., on the levels of serum glucose and triglyceride, fatty acid desaturation, and bile acid excretion in streptozotocin-induced diabetic rats. Agric Biol Chem. 1991;55(12):3005–10.
62. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–33. doi: https://doi.org/10.1210/jc.2006-1055
63. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. doi: https://doi.org/10.1016/j.diabres.2014.04.006
64. Nurcholis W, Munshif AA, Ambarsari L. Xanthorrhizol contents, α-glucosidase inhibition, and cytotoxic activities in ethyl acetate fraction of Curcuma zanthorrhiza accessions from Indonesia. Rev Bras Farmacogn. 2018;28(1):44–9. doi: https://doi.org/10.1016/j.bjp.2017.11.001
65. Oon SF, Nallappan M, Kassim NK, Shohaimi S, Sa’ariwijaya MSF, Tee TT, et al. Hypolipidemic activities of xanthorrhizol purified from centrifugal TLC. Biochem Biophys Res Commun. 2016;478(3):1403–8. doi: https://doi.org/10.1016/j.bbrc.2016.08.136
66. Rivera-Mancía S, Trujillo J, Chaverri JP. Utility of curcumin for the treatment of diabetes mellitus: evidence from preclinical and clinical studies. J Nutr Intermed Metab. 2018;14:29–41. doi: https://doi.org/10.1016/j.jnim.2018.05.001
67. Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, et al. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005;82(6):831–8. doi: https://doi.org/10.1002/jnr.20692
68. Weisler RH, Pandina GJ, Daly EJ, Cooper K, Gassmann-Mayer C. Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs. 2012;26(5):421–34. doi: https://doi.org/10.2165/11631990-000000000-00000
69. Nurhan AD, Gani MA, Budiatin AS, Siswodihardjo S, Khotib J. Molecular docking studies of Nigella sativa L and Curcuma xanthorrhiza Roxb secondary metabolites against histamine N-methyltransferase with their ADMET prediction. J Basic Clin Physiol Pharmacol. 2021;32(4):795–802. doi: https://doi.org/10.1515/jbcpp-2020-0425
70. Singh R, Ramakrishna R, Bhateria M, Bhatta RS. In vitro evaluation of Bacopa monniera extract and individual constituents on human recombinant monoamine oxidase enzymes. Phytother Res. 2014;28(9):1419–22. doi: https://doi.org/10.1002/ptr.5116
71. Khatri DK, Juvekar AR. Kinetics of inhibition of monoamine oxidase using curcumin and ellagic acid. Pharmacogn Mag. 2016;12(Suppl 2):S116–20. doi: https://doi.org/10.4103/0973-1296.182168
72. Khatri DK, Juvekar AR. Neuroprotective effect of curcumin as evinced by abrogation of rotenone-induced motor deficits, oxidative and mitochondrial dysfunctions in mouse model of Parkinson’s disease. Pharmacol Biochem Behav. 2016;150–1:39–47. doi: https://doi.org/10.1016/j.pbb.2016.09.002
73. Kim SH, Hong KO, Hwang JK, Park K-K. Xanthorrhizol has a potential to attenuate the high dose cisplatin-induced nephrotoxicity in mice. Food Chem Toxicol. 2005;43(1):117–22. doi: https://doi.org/10.1016/j.fct.2004.08.018
74. Crona DJ, Faso A, Nishijima TF, McGraw KA, Galsky MD, Milowsky MI. A systematic review of strategies to prevent cisplatin-induced nephrotoxicity. Oncologist. 2017;22(5):609–19. doi: https://doi.org/10.1634/theoncologist.2016-0319
75. Garg C, Sharma H, Garg M. Skin photo-protection with phytochemicals against photo-oxidative stress, photo-carcinogenesis, signal transduction pathways and extracellular matrix remodeling—an overview. Ageing Res Rev. 2020;62:101127. doi: https://doi.org/10.1016/j.arr.2020.101127
76. Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in ohotoaging. J Investig Dermatol Symp Proc. 2009;14(1):20–4. doi: https://doi.org/10.1038/jidsymp.2009.8
77. Li H, Gao A, Jiang N, Liu Q, Liang B, Li R, et al. Protective effect of curcumin against acute ultraviolet B irradiation-induced photo-damage. Photochem Photobiol. 2016;92(6):808–15. doi: https://doi.org/10.1111/php.12628
78. Park J-H, Jung Y-J, Antar?Aziz?Mohamed M, Hoon?Lee T, Lee C-H, Han D, et al. New bisabolane sesquiterpenes from the rhizomes of Curcuma xanthorrhiza Roxb. and their inhibitory effects on UVB-induced MMP-1 expression in human keratinocytes. Helv Chim Acta. 2014;97(3):438–46. doi: https://doi.org/10.1002/hlca.201300372
79. Draelos Z. Aquaporins: an introduction to a key factor in the mechanism of skin hydration. J Clin Aesthet Dermatol. 2012;5(7):53–6.
80. Yamazaki M, Maebayashi Y, Iwase N, Kaneko T. Studies on pharmacologically active principles from Indonesian crude drugs. I.: principle prolonging pentobarbital-induced sleeping time from Curcuma xanthorrhiza ROXB. Chem Pharm Bull. 1988;36(6):2070–4.
81. Devaraj S, Esfahani AS, Ismail S, Ramanathan S, Yam MF. Evaluation of the antinociceptive activity and acute oral toxicity of standardized ethanolic extract of the rhizome of Curcuma xanthorrhiza Roxb. Molecules. 2010;15(4):2925–34. doi: https://doi.org/10.3390/molecules15042925
82. Listyawati S. Toxicity studies of the rhizome Curcuma xanthorrhiza Roxb. and Curcuma zedoaria Roscoe on hematological and male reproduction system of mice (Mus musculus L.). Asian J Nat Prod Biochem. 2006;4(1):10–3.
83. Irsyad MN, Jumari J, Murningsih M. Ethnobotany study of rural community Sukolilo, Kendeng Mountains, Pati, Central Java. Bioma. 2013;15(1):27–34.
84. Arum GPF, Retnoningsih A, Irsadi A. Etnobotani tumbuhan obat masyarakat desa keseneng kecamatan Sumowono kabupaten Semarang Jawa Tengah. Unnes J Life Sci. 2012;1(2):1–7.
85. Luo C, Xu X, Wei X, Feng W, Huang H, Liu H, et al. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol Res. 2019;148:104409. doi: https://doi.org/10.1016/j.phrs.2019.104409
86. Jinyuan S, editor. Effects of curcumin on physical fatigue and oxidative damage in forced swimming mice. E3S Web Conf. 2020;189:02021.
87. Hamidie RDR, Yamada T, Ishizawa R, Saito Y, Masuda K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism. 2015;64(10):1334–47. doi: https://doi.org/10.1016/j.metabol.2015.07.010
88. Huang W-C, Chiu W-C, Chuang H-L, Tang D-W, Lee Z-M, Wei L, et al. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients. 2015;7(2):905–21.