INTRODUCTION
Osteosarcoma (OS) is a bone cancer that begins with a tumor secreted by the abnormal growth of osteoid substances and immature bone [1]. The overall and event-free survival rates over 5 years are approximately 71% and 54%, respectively [2]. OS primarily affects adolescents aged 15–19 [3] and elders over 65 [4]. Although OS is a rare type of childhood cancer, it accounts for 3%–5% of children’s carcinomas and nearly 0.2% of all malignant tumors [5]. It has been revealed that 25% of them first seek medical help with metastases [6]. For adolescents with metastases, the 5-year survival rate drops to 70% and decreases to 20%–30% [1]. Due to its recurrence or post-metastasis [7], the overall and event-free survival rates over 5 years are 38.1%? ± ?6.4% and 25?% ±?5.3%, respectively [8]. Around 15%–20% of these cases [9] had the pulmonary metastases tree [8], which causes a mortality rate of more than 90% [10]. As a result, only about 20%–30% of such cases live for a prolonged period, compared with 65%–70% of localized cases [11].
Over nearly four decades, scientists and clinicians have attempted to solve the 30% ineffectiveness problem [12] for the standard treatments [13,14], such as chemotherapy [15], immunotherapy [16], inhibitory therapy [17], and surgery [18], resulting in progression to malignant tumors [19]. Even after numerous trials, there has been no significant improvement. This is due to the immunotherapy on tumorigenesis [20], which develops severe immunosuppression and chemoresistance after long-term treatments [21–23] . Furthermore, the innate and acquired nature of chemoresistance in the tumor microenvironment (TME) eventually causes the therapy’s progression to stall [24]. Thus, this primary barrier needs to be encountered by intensifying more efficacious therapies with higher cure rates [25]. However, not much research provides an in-depth understanding of the relationship between tumorigenesis, metastasis, immune evasion, and chemoresistance. As a result, the efforts in de-signing an efficacy, long-term use [26], and personalized precision medicine [27] for combinational and targeted therapy [28–30] remain inadequate [31].
Because the four stages are intertwined and complex [32], this review focuses on clarifying their relationship and encounter therapies. First of all, the status quo of OS therapies is presented, along with their historical development and clinical advancements. A comprehensive timeline is drawn to demonstrate significant discoveries and advancements in OS studies. Besides, the summary of completed years of sarcoma clinical trials is tabulated to highlight the seminal discoveries and major clinical triumphs. In the content, the bi-directional relationship between these four OS mutation stages—tumorigenesis, metastasis, immune evasion, and chemoresistance—is clearly described. Further, short and precise definitions are given to each of them to reach a common understanding. Hereafter, their intertwined therapies could be discussed individually. Herein, it is notable that many clinically relevant therapies nowadays are combinational and multifunctional in order to cure the complex OS stages. Notably, intertwined therapy is a fact that should not be overlooked; however, it is prudent to discuss them by reconstructing them to elaborate precisely. Through this review, the stages and therapies of OS are precisely defined and clearly elucidated, which are expected to serve as guidance for scientists and clinicians.
STATUS QUO OF OS THERAPIES
Historical development
The significant discoveries and advancements in OS studies are shown as a timeline in Figure 1. The first in vivo test was the characteristic investigation of the fresh anterior lobe effects in 1922 by Evans and Long [33]. After 42 years, the first gene for human growth hormone (HGH) was cloned by Li and Liu [34]. In 1978, the first high-dose methotrexate (Mtx) for OS treatment was introduced by Jaffe et al. [35]. Later in 1982, the compliance issue of high dosage for preoperative adjuvant chemotherapy was addressed by Rosen et al. [36]. After 2 years, the biological and immunological properties of OS were investigated by Zapf et al. [37] using insulin-like growth factors (IGF). However, the first in vitro test was done in 1987 by Stashenko et al. [38] who investigated a bone inhibitor using Interleukin (IL)-1β. Six years later, the first recombinant technology for HGH was successfully developed by Bengtsson et al. [39]. In 1997, the first common childhood use of combined chemotherapy with etoposide and ifosfamide (EnI) was developed by Gentet et al. [40]. McGary et al. [41] were the first to use the Tyrosine Kinase Inhibitor STI571 in 2002 for targeted OS therapy. Further works by Nardin et al. [42] used liposomal muramyl tripeptide phosphatidylethanolamine in immunotherapy to target and activate macrophages. Nevertheless, Tang et al. [43] introduced the stem cell with a salinomycin inhibitor in 2011. Finally, the first preliminary efficacy and safety drug carrier with a nab-sirolimus was introduced in 2019 by Gordon et al. [44].
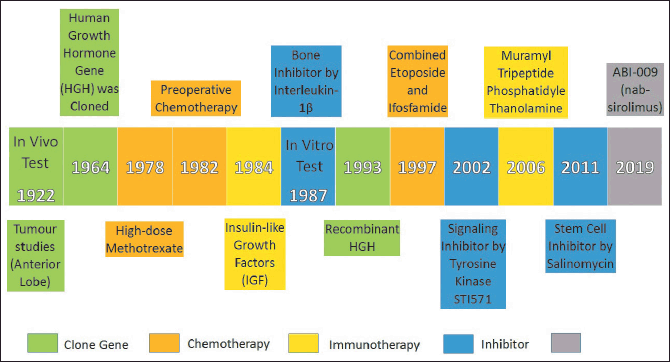 | Figure 1. Timeline of significant discoveries and advancements in OS studies. [Click here to view] |
Clinical advancement
OS research is extremely hard and remains a global challenge. Despite the fact that many clinical trials had begun, the majority of them could not be completed. For the past 20 years, only ten clinical trials with United States federal government clinical trial identifiers (GCTI) have been successfully completed. Thus, the sarcoma types completed their clinical trials in years with active pharmaceutical ingredients (API) and primary tests, as shown in Table 1. For all these trials, there are only two main types of OS: soft and solid, as observed in the table. The following API are appropriate for OS: topotecan (Tpt) [45], pazopanib (Pzp) [46], placebo (Plb) [47], gemcitabine (Gct) [48], M6620 [49,50], regorafenib (Rgf) [51,52], glembatumumab vedotin (GV) [53,54], lenvatinib [55], EnI [56], nab-rapamycin (Rpm) [57,58], cyclophosphamide (Cfa) [59], simvastatin (Sim) [60], myeloid growth factor (MGF) [61], nab-paclitaxel [62,63], Mtx [64], and doxorubicin (Dox) [65]. Lastly, only two types of primary tests were successfully conducted, such as laboratory biomarker analyses [66] and dose escalation studies [67].
OS BIDIRECTIONAL MUTATION STAGES
In all kinds of OS or human carcinoma, the TP53 gene is mutated (somatic mutations) in more than 50% of cases. The DNA-binding domain is principally mutated. Other than this site, 20% of cases mutated [68,69] . Thereby, TP53 gene mutations have remained prospective diagnostic components, which can, to a greater extent, increase the precision of forecasting continuity of life and cancer-free longevity among patients with carcinoma [69,70]. The carcinogenic activity of mutant TP53 is almost indistinguishable in sarcoma and multiple other neoplastic diseases [71]. Multiple appraisal techniques were applied among OS cases, revealing that the TP53 gene was lost in the presence of two different alleles. Consequently, there is frequent demand for an up-grade in chemoresistance to achieve chemotherapeutic efficacy [72].
OS therapies are difficult because they progress and reverse through four mutation stages and are inter-twined, including TME, metastasis, immune evasion, and chemotherapeutic resistance, as shown in Figure 2. The bidirectional complexity of progression and reversion in OS mutation stages is influenced by the exosomes of a tumor, stem, mesenchymal, immune, fibroblast, and endothelial cells [73]. There is mounting evidence that signal molecules such as neurotransmitters, enzymes, hormones, and nucleic acids [74] are involved in the angiogenesis, growth, migration, metastasis, and apoptosis of the above-mentioned cells, involving intercellular cell communication, body regulation, and immune responses [75]. In this cellular communication, extracellular vesicles (EV) play a key role [76], which could be derived from various cells such as OS cells, mesenchymal stem cells (MSC), adipose-derived MSC (ADMSC), cancer-associated stromal fibroblasts (CAF), and macrophages [77]. These EVs regulate the activity of recipient cells, including angiogenesis, proliferation, invasion, migration, metastasis, chemotherapeutic resistance, and apoptosis, by using their cargoes of proteins, DNA, and RNA [78]. These three cargoes have distinct metabolic dynamics [79] including a connection with the EV components’ biogenesis machinery, a cellular homeostasis regulator with cytoplasmic DNA sensor activation, and parental cell function efficiency at different states [80]. The creation of biomarker vehicles [81] that employ the aforementioned protumorigenic components and signaling pathways to circulate immune responses from OS cancer diseases remains a significant clinical trial challenge [82].
Tumorigenesis
TME is composed of EV secretion cells, MSC, and tumor cells. The EV cells are secreted by MSC and immune cells to alter macrophage phenotype-2 (M2) [83] and regulate tumor progression via the Wnt signaling pathway [84]. Furthermore, the EV cells are the paracrine factors secreted by human bone marrow MSC (BMSC), such as osteoclasts, osteoblasts, and endothelial cells, to regulate tumor cells via communication with the Hedgehog signaling pathway [85]. As a result, the M2 and tumor cells regulate TME by EV secretions in order to promote angiogenesis, growth, and metastasis [86,87]. Tumorigenesis research in TME is concerned with how coexisting cells interact and communicate with one another.
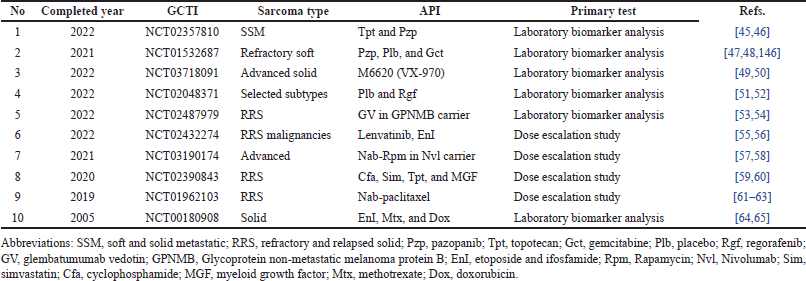 | Table 1. Summary of completed years for sarcoma clinical trials with their types, API, and primary tests. [Click here to view] |
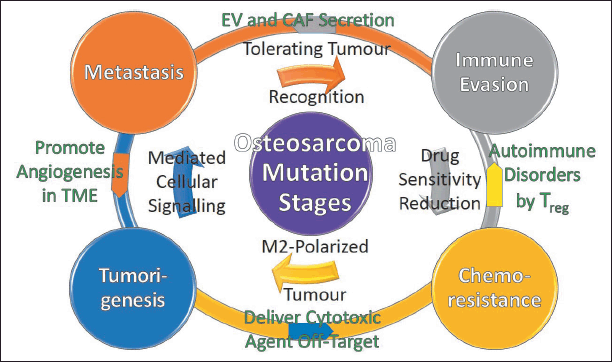 | Figure 2. Schematic of bidirectional OS mutation stages. [Click here to view] |
Metastasis
The metastasis potential is affected by the communication between the stressed MSC and the micro RNA (miRNA) content of EV. Tumor cells metastasize in three ways: by secreting EV, by influencing TME, and by mediating the transformation of distant MSC. Direct EV secretion by osteoblasts and CAF can improve migrability. The induction of metastasis can be influenced by regulating tumor and MSC oncogenic phenotypes in TME. The pro-angiogenic factors from endothelial cells can mediate EV transformation to modulate cell invasiveness and promote metastasis. Metastasis could be activated either by modulating tumor-associated macrophage (TAM) cellular signaling to promote the M2 or by producing transforming growth factor beta (TGFβ)-2 to create an immunosuppressive and pro-TME [88]. As a result, the tumor cells could be metastasized by inducing a pro-metastatic and tumorigenic phenotype and mediating transformation into local or distant cells.
Immune evasion
The immune system is divided into innate and adaptive immunizations, which are always related to the bone microenvironment [89]. The immune evasion occurred because the inefficient immune cells allowed the tumors to evade the immune surveillance systems or the host immune checkpoint through multiple mechanisms [24]. This inefficiency induced a tolerance for the T-cell receptor (TCR), resulting in a dormant response to tumor recognition [90]. Therefore, the tumor cancer cells in TME escaped immunotherapy. However, this peripheral tolerance of host-cell immune responses is protected by regulating T regulatory cells (Treg) to prevent autoimmune disorders [91]. In fact, two major mechanisms induce immune tolerance [92]: T-cell-mediated inflammation suppression and no tumor signals received by the major histocompatibility complex antigen presentation [93]. Traditionally, the plasma protease thrombin cleaves glycoprotein A repetitions predominant in tumor immune evasion to release active TGFβ [94]. TGFβ is the main coordinator and mediator between both mechanisms mentioned in immune evasion [95]. TGFβ increased programmed cell death protein (PD) ligand-1 expression on TAM [96] to bind with PD-1 (CD279) for cytotoxic T lymphocyte-associated anti-gen (CTLA)-4 inhibition. CTLA4 (CD152) is a membrane glycoprotein of immunosuppressive Treg that binds to costimulatory molecules CD80 and CD86 to inhibit early T cell (CD8+ and CD4+) activation [97]. These T cells are anti-tumor cells that respond to CAF for immune evasion regulation [98].
Chemoresistance
Chemoresistance is chemotherapeutic resistance, resulting in a chemotherapeutic efficacy deficit [99]. It always results in cytotoxic agents being minimally delivered or severely off-target, destroying therapeutic compliance effects [100]. Chemoresistance in cancer cells can be either inherent or acquired, with the latter increasing proportionally with the duration of the therapy [101]. Chemoresistance is commonly known as multidrug resistance (MDR), which is drug resistance to Mtx, Dox, and cis-diamminedichloroplatinum (II) (CDDP) drugs [102]. Drug accumulation in clones and stem cells altered TME, leading to mutation and decreased drug sensitivity [103]. For instance, chemoresistance decreased Dox sensitivity, resulting in M2 induction, which caused tumor cells to spread without responsiveness to Dox [104]. However, the sensitivity of drugs can be induced by the transfer of specific bioactive molecules, such as non-coding RNA and proteomic signatures [105].
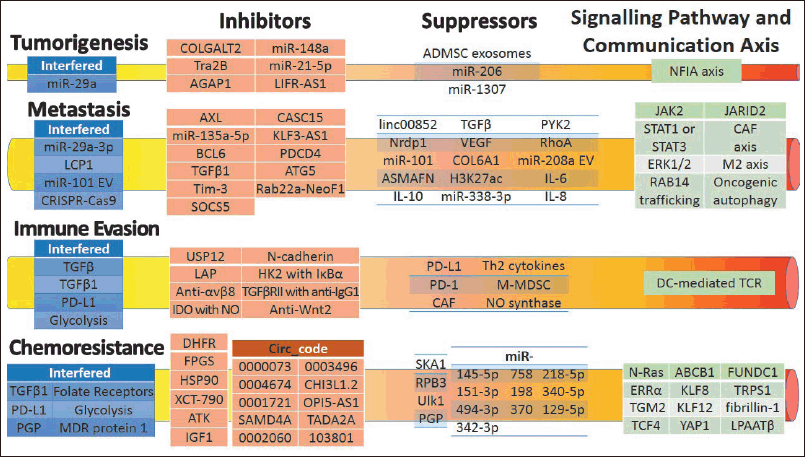 | Figure 3. Schematic of recent OS medicines and their therapy mechanisms for tumorigenesis, metastasis, immune evasion, and chemoresistance. [Click here to view] |
RECENT OS THERAPIES
Because there have been numerous OS therapies over the last four decades, only the five most recent years are considered below. Although many OS therapies have been developed, their individual and combinational mechanisms are dispersed [106]. Therefore, a schematic is drawn to elucidate their recent medicines and therapy mechanisms in OS, as shown in Figure 3. Medicines are used to inhibit and suppress tumorigenesis, metastasis, immune evasion, and chemoresistance via communication mediums [107]. Targeted therapies can be developed to intensify the therapies and achieve higher cure rates by thoroughly understanding the roles of genes in communication axes and signaling pathways [108].
Tumorigenesis therapies
The tumorigenesis therapies are generally medicated in connection with suppressive, regulative, and inhibitive treatment mechanisms [109]. There is a summary of five recent studies that have addressed tumorigenesis with medicines for their treatment mechanisms, as shown in Table 2. For instance, three studies used suppressive mechanism treatments to halt tumorigenesis’ proliferation, migration, and invasion. Collagen beta (1-O) galactosyl transferase 2 (COLGALT2) inhibitor [110,111], transformer 2β (Tra2B) [112,113], and ArfGAP with GTPase domain 1 (AGAP1) [114,115] were used as the medicines to suppress ADMSC exosomes, miR-206, and miR-1307, respectively. In studies of chondrogenesis like osteoclast differentiation and bone resorption activity, the miR-148a and miR-21-5p EVs were used to increase their genes to mimic umbilical vein endothelial cell (UVEC) formation in TME [86,116]. Furthermore, long non-coding RNA (lncRNA) leukemia inhibitory factor receptor antisense RNA1 (LIFR-AS1) inhibitor was used to inhibit miR-29a in the nuclear factor IA (NFIA) axis to suppress human peripheral-blood monocytes-induced macrophage-derived exosomes [115,117]. All 10 studies are related to exosomal-derived genes, and these could be used as diagnosis and prognosis markers in OS progression [118].
Interfere communication mediators’ therapies
Metastasis is stimulated and controlled by intercellular communication in endothelial cells [119]. Both stages can be inhibited by interfering with their direct and indirect communication mediators against endothelial changes [120]. There is a summary of 20 recent studies that have addressed metastasis with medicines that interfere with communication mediators in signaling pathways, as shown in Table 3. For instance, 12 studies interfered with communication mediators using inhibitors, including AXL receptor tyrosine kinase (AXL) [121,122], miR-135a-5p [123,124], messenger RNA (mRNA) B-cell lymphoma-6 (BCL6) [125,126], TGFβ1 [127,128], T-cell immunoglobulin and mucin-domain containing protein-3 (Tim-3) [129,130], and suppressor of cytokine signaling-5 (SOCS5) [131,132]. These inhibitors interfered with miR-29a-3p, BMSC-derived exosomal lymphocyte cytosolic protein-1 (LCP1), miR-101 EV, CRISPR-associated protein-9 (Cas9), M2 mediation, and signal transducers and activators of transcription (STAT)-1 mediation by suppressing long intergenic non-protein coding RNA (linc)-00852 in the jumonji and AT-rich interaction domain containing 2 (JARID2) axis; neuregulin receptor degradation protein-1 (NRDP1) in the Janus kinase-2 (JAK2)/STAT3 signaling path-way; ADMSC-derived miR-101; CAF and α-smooth muscle actin expression and fibronectin (ASMAFN) differentiation; IL-10, TGFβ, and vascular endothelial growth factor (VEGF) secretions; and collagen type VI alpha 1 (COL6A1) from histone3 lysine27 acetylation (H3K27ac) activated in CAF conversion with IL-6 and IL-8 secretions; respectively.
The other eight studies interfered with communication mediators using other medicines, such as cancer susceptibility 15 (CASC15) or Krüppel-like factor 3 antisense RNA 1 (KLF3-AS1) [133,134], programmed cell death 4 (PDCD4) [135,136], autophagy-related gene 5 (ATG5) [137,138], and Rab22a-NeoF1 fusion protein [139,140]. Other medicines interfered with Ras-associated binding 14 (RAB14), extracellular signal-regulated kinase-1/2 (ERK1/2) signaling pathway, oncogenic autophagy, and M2 with Arginylglycylaspartic acid (RGD) peptide internalization in STAT3 by suppressing miR-338-3p; miR-208a EV; BMSC-derived EV; and protein tyrosine kinase-2 (PYK2) and Ras homolog family member A (RhoA); respectively. All of the medicines used in the 20 studies interfered with communication mediators related to EV secretions or protein expression.
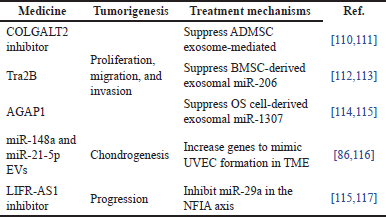 | Table 2. Summary of medicines for tumorigenesis with treatment mechanisms. [Click here to view] |
Immune evasion therapies
The suppression and communication barriers of immune cells allow immune evasion [141]. Tumour cells escape being destroyed by the immune system because the immune cells, such as neutrophils, monocytes, macrophages, dendritic cells, natural killer cells, and B and T lymphocytes, are suppressed [142]. Besides, the tumor’s immune responses are barred from immune checkpoint activations, thereby causing immune evasion [143]. Therefore, immune evasion is eliminated by suppressing and inhibiting both mechanisms. There is a summary of 20 recent studies with medicines and prevention mechanisms for immune evasion, as shown in Table 4. For instance, eight studies used medicines to suppress immune evasion mechanisms, such as mRNA N-cadherin [144,145], ubiquitin-specific peptidase 12 (USP12) inhibitor [146,147], latency-associated peptide domain (LAP) inhibitor [148,149], and anti-Wnt2 mAb [98,150] . These medicines suppressed PD-L1, PD-1, monocytic myeloid-derived suppressor cells (M-MDSC), NO synthase, and CAF in order to activate CD8+ T cells and TCR. The remaining eight studies used medicines to inhibit immune evasion mechanisms, such as anti-αvβ8 integrin [151,152], hexokinase-2 (HK2)-mediated phosphorylation of i-kappa-b-alpha (IκBα) [153,154], indoleamine 2,3-dioxygenase (IDO) inhibitor with NO [155,156], and TGFβ receptor II (TGFβRII) with anti-IgG1 (also known as bintrafusp alfa) [157,158]. These medicines inhibited the expression of TGFβ, TGFβ1, PD-L1, and glycolysis in order to activate CD8+ T cells and Treg cells. In all 20 studies, ignoring the immune evasion due to innate and adaptive immunizations, the medical therapies focus on activating immune cells, such as CD8+ T cells, TCR, and Treg cells. As a result, immune evasion is prevented by checkpoint blockade therapy [159], such as PD-L1, PD-1, IL-4, IL-10, M-MDSC, NO synthase, CAF, TGFβ, and TGFβ1 [160].
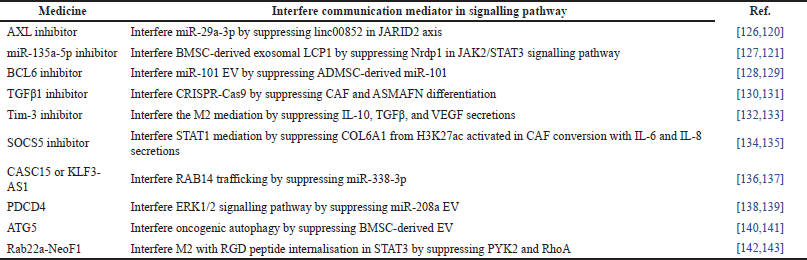 | Table 3. Summary of medicines that interfere communication mediators’ therapies. [Click here to view] |
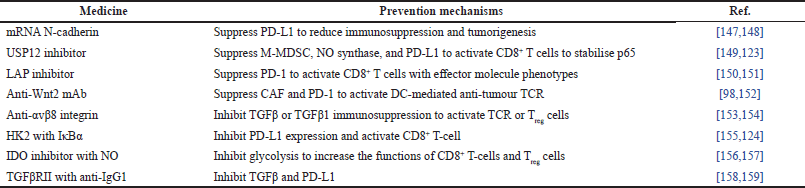 | Table 4. Summary of immune evasion medicines with their prevention mechanisms. [Click here to view] |
Chemoresistance therapies
Chemoresistance therapies are always accompanied by treatments for severe off-target effects to restore therapeutic compliance [161]. The acquired nature of chemoresistance in cancer cells will minimize the cytotoxic agent’s delivery proportionally to the chemotherapeutic treatment duration [162]. Hence, this becomes a primary challenge for therapeutic agents and cellular lesions in OS therapy [8]. Drug accumulation in cells, intracellular detoxification, apoptosis, DNA repair, signal transduction disruption, and tumor stem cell immunity all contribute to chemoresistance [163]. Most therapeutic approaches involve inhibition of the oncogene’s expression to interfere with or mute the communication pathways or axis [164]. Some use drug carriers to avoid rapid drug clearance and prolong release [165,166] . As a result, chemoresistance therapies should focus on oncogene inhibition, drug influx and efflux, and drug carriers [167].
Chemoresistance therapies are divided into two types: inhibitor therapies and gene knockdown therapies. There is a summary of ten recent studies that used inhibitor therapies for different types of drug resistance and their chemoresistance prevention in OS cells, as shown in Table 5. For instance, there are four studies focused on reducing folate receptors for Mtx and Dox drug resistance. Dihydrofolate reductase (DHFR) [168,169] and folylpoly-γ-glutamate synthetase (FPGS) [170,171] inhibitors were used to induce cancer cell apoptosis and inhibit the interaction of spindle and kinetochore associated complex subunit 1 (SKA1) and RNA polymerase II subunit 3 (RPB3), respectively. DHFR reduced the affinity of Mtx resistance by converting dihydrofolate to tetrahydrofolate in order to inhibit purine and thymidine synthesis, resulting in a deficit in DNA replication and apoptosis. Another two studies of (CDDP or cisplatin) drug resistance used the heat shock protein (HSP)-90AA1 gene inhibitor [85,172] to deactivate autophagy activating kinase 1 (Ulk1) in FUN14 domain-containing protein 1 (FUNDC1) mediation for mitophagy activation to induce apoptosis. Mitophagy is mitochondrial removal through autophagy, which allows tumor cells to survive cellular stress by clearing damaged organelles and proteins. Two studies of P-glycoprotein (PGP) were inhibited by the inverse agonist XCT-790 or the anlotinib tyrosine kinase (ATK) inhibitor [173,174] for mRNA ATP-binding cassette subfamily B member 1 (ABCB1) in the estrogen-related receptor alpha (ERRα) axis. Another two studies of ABCB1 in the ERRα axis were inhibited by IGF-1 [175,176] in order to reverse metabolic disorders. As a result, folate receptors, FUNDC1-mediated Ulk1, and ABCB1 in the ERRα axis are the key targets in chemoresistance therapies.
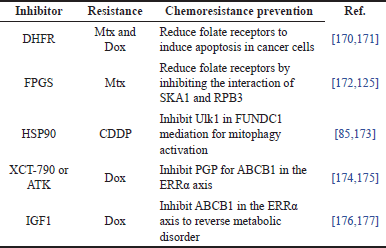 | Table 5. Summary of inhibitors, drug resistance, and their chemoresistance prevention in OS cells. [Click here to view] |
Despite the above key targets in chemoresistance therapies, the expression of siRNA oncogenes has been popularly used recently to interfere with or mute the communication pathways or axis [177]. There is a summary of 20 recent studies that used siRNA gene knockdown therapies for different types of drug resistance and their chemoresistance prevention in OS cells, as shown in Table 6. Transmitting circular RNA (circ_) is used to prevent the Mtx, Dox, and CDDP drug resistance in the 4, 10, and 6 studies, respectively. For Mtx instances, the circ_0000073 [178,179] and circ_0081001 [180,181] gene knockdowns inhibited the N-Ras pathway by sponging miR-145-5p and miR-151-3p and the transglutaminase-2 (TGM2) axis by miR-494-3p, respectively. For Dox instances, the gene knockdowns of circ_0004674 [182,183], circ_0001721 [184,185], circ_SAMD4A (sterile alpha motif domain) [186,187], circ_0002060 [188,189], and circ_0003496 [190,191] inhibited the fibrillin-1 axis by sponging miR-342-3p, the transcription factor 4 (TCF4) axis by miR-758, the Krüppel-like factor (KLF)-8 axis by miR-218-5p, the ABCB1 axis by miR-198, and the KLF12 axis by miR-370, respectively. For CDDP instances, the gene knockdowns of circ_CHI3L1.2 (chitinase 3-like 1.2) or lncRNA OPI5-AS [192,193], circ_transcriptional adaptor 2A (TADA2A) [194,195], and circ_103801 [196,197] inhibited the lysophosphatidic acid acyltransferase β (LPAATβ) axis by sponging miR-340-5p, the yes-associated protein (YAP) and trichorhinophalangeal syndrome 1 (TRPS1) axis by miR-129-5p, and the MDR-associated protein 1 and PGP, respectively. As a result, the communication pathways of chemoresistance in OS cells would be more effectively prevented by the therapies targeting oncogene expression with their knockdowns.
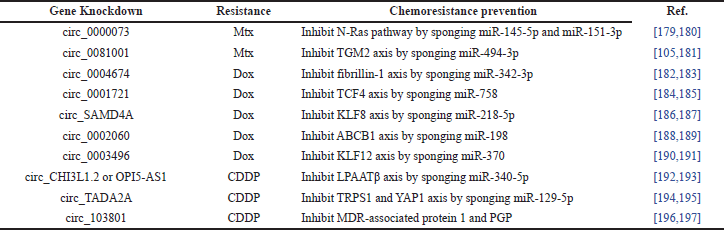 | Table 6. Summary of gene knockdowns, drug resistance, and their chemoresistance prevention in OS cells. [Click here to view] |
CONCLUSION
Despite the innate and acquired nature of OS, its progression is intertwined, including cycles of tumorigenesis, metastasis, immune evasion, and chemoresistance. Firstly, tumorigenesis is the result of M2 alterations, which are progressed via signaling pathways by the MSC- and immune cell-secreted EV. Secondly, metastasis is potentially affected by the communication between the stressed MSC and the miRNA content of EV. Thirdly, immune evasion occurred because tumor cells evaded the host immune checkpoint through the TCR tolerance mechanism, resulting in Treg in autoimmune disorders. Finally, chemoresistance causes cytotoxic agents to be delivered severely off-target, resulting in a chemotherapeutic efficacy deficit. These four stages of progression are treated by the combinational and multifunctional therapies listed below. Five tumorigenesis therapy studies have been conducted using medicines such as COLGALT2 inhibitors, Tra2B, and AGAP1, miR-148a and miR-21-5p EVs, and the lncRNA LIFR-AS1 inhibitor. The mechanisms of tumorigenesis were being suppressed, regulated, and inhibited, such as proliferation, migration, invasion, chondrogenesis, and UVEC formation. Their targets include ADMSC exosomes, miR-206, miR-1307, miR-148a, miR-21-5p, and miR-29a in the NFIA axis. Metastasis therapies are treated with medicines related to EV secretions and protein expression for intercellular communication in endothelial cells. There have been 20 therapy studies using inhibitor and disruptor medicines to inhibit protumorigenic expression and disrupt signaling pathways. AXL, miR-135a-5p, mRNA BCL6, TGFβ1, Tim-3, and SOCS5 are the inhibitor medicines. These medicines inhibit miR-29a-3p and linc-00852 in the JARID2 axis, LCP1 and NRDP1 in the JAK2/STAT3 signaling pathway, miR-101 EV, Cas9 in CAF and ASMAFN differentiation, IL-10, TGFβ, and VEGF secretions for M2, and CAF conversion with COL6A1 and H3K27ac in the STAT1 signaling pathway. CASC15, KLF3-AS1, PDCD4, ATG5, and Rab22a-NeoF1 are the disruptor medicines. These medicines disrupt RAB14 by miR-338-3p, the ERK1/2 signaling pathway by miR-208a EV, oncogenic autophagy by BMSC-derived EV, and M2 with RGD in STAT3 by PYK2 and RhoA. Immune evasion is treated by activating CD8+ T cells and connecting TCR and Treg cells for immune checkpoint activations and communication checkpoint regulations, respectively. Sixteen therapy studies have been conducted using medicines to suppress and inhibit immune evasion mechanisms. These medicines are mRNA N-cadherin, USP12 inhibitor, LAP inhibitor, anti-Wnt2 mAb, anti-αvβ8 integrin, HK2-mediated IκBα, IDO inhibitor with NO, and TGFβRII with anti-IgG1. Medical targets include PD-L1, PD-1, M-MDSC, NO synthase, CAF, TGFβ, and TGFβ1. Chemoresistance therapies use oncogene inhibition as well as drug carriers for influx and efflux to repair immune therapies and disrupt communication pathways. There are 30 studies of chemoresistance therapies focused on Mtx, Dox, and CDDP drug resistance by using inhibitor therapies and gene knockdown therapies. The inhibitors are DHFR, FPGS, HSP-90AA1, XCT-790, ATKI, and IGF1. The inhibitor targets folate receptors, FUNDC1-mediated Ulk1, and ABCB1 in the ERRα axis. Besides, the gene knockdowns are circ_0000073, circ_0081001, circ_0004674, circ_0001721, circ_SAMD4A, circ_0002060, circ_0003496, circ_CHI3L1.2 or lncRNA OPI5-AS, circ_TADA2A, and circ_103801. The gene knockdown’s targets are miR-145-5p and miR-151-3p in the N-Ras pathway, miR-494-3p in the TGM2 axis, miR-342-3p in the fibrillin-1 axis, miR-758 in the TCF4 axis, miR-218-5p in the KLF-8 axis, miR-198 in the ABCB1 axis, miR-370 in the KLF12 axis, miR-340-5p in the LPAATβ axis, miR-129-5p in the YAP/TRPS1 axis, and the MDR-associated protein 1 and PGP. In conclusion, all these OS therapies are individually elucidated to treat tumorigenesis, metastasis, immune evasion, and chemoresistance. However, their OS mutation stages are bidirectional and intertwined, resulting in their being combinational and multifunctional.
CHALLENGES AND FUTURE
OS is an unusual and complicated malignant tumor that necessitates an integrative and interdisciplinary therapeutic approach [198–200] . It has been reported that a multidisciplinary approach requires collaboration and cooperation between pediatric or medical cancer specialists, surgeons, pathologists, psychiatrists, radiologists, and radiotherapists [5]. Thus, several models of OS neoplasm have diverse clinical outcomes [201], making the diagnosis and treatment of OS cancer extremely challenging. Thereby, the therapeutic regimen for OS patients has not been systematized, harmonized, or standardized [202]. It has been reported that thorough surgical eradication of all sites of primary and metastatic OS is obligatory, foretelling better clinical end results and continuity of quality life [5]. However, those OS cases have several primary and metastatic OS disease locations that are not manageable for total surgical resection and result in impecunious clinical consequences. Furthermore, preoperative chemotherapies cause chemoresistance, resulting in a two-fold increase in the cisplatin capability of mutational load in OS cases [71]. As a result, chemotherapeutic regimens for recurring or replacement cases of chemotherapy resistance are constantly being improved [203].
According to a recent study, the genetic framework and oncogenesis process of OS are largely unknown, which is impeding research efforts. The immune microenvironment of OS tumors has been extensively studied. It found that OS possesses noticeable diversity and a complicated all-around mode of process regarding malignancy continuation and metastasis [142]. Another study reported that MDSCs massively invade OS tumors and promote anti-cancer immune-suppressive activities [142,204,205]. Research studies said that preoperative chemotherapy agents, e.g., Dox, CDDP, and ifosfamide, effectively brought down the MDSC count in OS cases and, after that, augmented both immune sensitivities and the overall immune system [206]. Metformin has been shown in studies to effectively reduce OS tumor progression and size. Metformin also shows substantial activity in reducing polymorphonuclear MDSC; nevertheless, no considerable variability was observed for M-MDSC [207]. Sodium-glucose cotransporter 2 (SGLT2) is a principal intercessor of epithelial glucose transport. It has been proclaimed that SGLT2 is vigorously and exaggeratedly exhibited in several malignant tumor cells, including OS [208]. Antagonizing overexpressed SGLT2 appreciably hinders cancer advancement, e.g., breast cancer, cervical cancer, hepatocellular cancer, prostate cancer, and lung cancer [209]. Although the antimalignant pharmacodynamics of SGLT2 antagonists in OS malignancy remain imprecise [208–210]. This narrative review advocates more research regarding this malignancy and safeguards our children and adults from the atrocities of this cancer.
LIST OF ABBREVIATIONS
ABCB1, ATP-binding cassette subfamily B member 1; ADMSC, adipose-derived MSC; AGAP1, ArfGAP with GTPase domain 1; API, active pharmaceutical ingredients; ASMAFN, α-smooth muscle actin expression and fibronectin; ATK, anlotinib tyrosine kinase; ATG5, autophagy-related gene 5; AXL, AXL receptor tyrosine kinase; BCL6, B-cell lymphoma-6; BMSC, bone marrow MSC; CAF, cancer-associated stromal fibroblasts; Cas9, CRISPR-associated protein-9; CASC15, cancer susceptibility 15; CDDP, cis-diamminedichloroplatinum (II); Cfa, cyclophosphamide; CHI3L1.2, chitinase 3-like 1.2; COL6A1, collagen type VI alpha 1; circ_, circular RNA; COLGALT2, Collagen beta (1-O) galactosyl transferase 2; CTLA, cytotoxic T lymphocyte-associated antigen; DHFR, Dihydrofolate reductase; Dox, doxorubicin; EnI, etoposide and ifosfamide; ERK1/2, extracellular signal-regulated kinase-1/2; ERRα, oestrogen-related receptor alpha; EV, extracellular vesicles; FPGS, folylpoly-γ-glutamate synthetase; FUNDC1, FUN14 domain-containing protein 1; Gct, gemcitabine; GCTI, United States federal government clinical trial identifiers; GPNMB, Glycoprotein non-metastatic melanoma protein B; GV, glembatumumab vedotin; H3K27ac, histone3 lysine27 acetylation; HGH, human growth hormone; HK2, hexokinase-2; HSP, heat shock protein; IDO, indoleamine 2,3-dioxygenase; IGF, insulin-like growth factors; IκBα, phosphorylation of i-kappa-b-alpha; IL, Interleukin; JAK2, Janus kinase-2; JARID2, jumonji and AT-rich interaction domain containing 2; KLF, Krüppel-like factor; KLF3-AS1, Krüppel-like factor 3 antisense RNA 1; LAP, latency-associated peptide domain; LCP1, lymphocyte cytosolic protein-1; LIFR-AS1, leukaemia inhibitory factor receptor antisense RNA1; linc, long intergenic non-protein coding RNA; lncRNA, long non-coding RNA; LPAATβ, lysophosphatidic acid acyltransferase β; M-MDSC, monocytic myeloid-derived suppressor cells; M2, macrophage phenotype-2; MDR, multidrug resistance; MGF, myeloid growth factor; miRNA, micro RNA; mRNA, messenger RNA; MSC, mesenchymal stem cells; Mtx, methotrexate; NFIA, nuclear factor IA; NRDP1, neuregulin receptor degradation protein-1; OS, osteosarcoma; PD, programmed cell death protein; PDCD4 programmed cell death 4; PGP, P-glycoprotein; Plb, placebo; PYK2, protein tyrosine kinase-2; Pzp, pazopanib; Rgf, regorafenib; RAB14, Ras-associated binding 14; RGD, Arginylglycylaspartic acid; RhoA, Ras homolog family member A; RPB3, RNA polymerase II subunit 3; Rpm, Rapamycin; RRS, refractory and relapsed solid; SAMD4A, sterile alpha motif domain; Sim, simvastatin; SKA1, spindle and kinetochore associated complex subunit 1; SOCS5, suppressor of cytokine signalling-5; SSM, soft and solid metastatic; STAT, signal transducers and activators of transcription; TADA2A, transcriptional adaptor 2A; TAM, tumour-associated macro-phage; TCF4, transcription factor 4; TCR, T-cell receptor; TGFβ, transforming growth factor beta; TGFβRII, TGFβ receptor II; TGM2, transglutaminase-2; Tim-3, T-cell immunoglobulin and mucin-domain containing protein-3; TME, tumour microenvironment; Tpt, topotecan; Tra2B, transformer 2β; Treg, T regulatory cells; TRPS1, trichorhinophalangeal syndrome 1; Ulk1, autophagy activating kinase 1; USP12, ubiquitin-specific peptidase 12; UVEC, umbilical vein endothelial cell; VEGF, vascular endothelial growth factor; and YAP, yes-associated protein.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The work was funded by the Ministry of Higher Education (MOHE) Malaysia, via the Fundamental Research Grant Scheme (FRGS), grant number FRGS/1/2021/STG01/UPNM/01/1.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Moukengue B, Lallier M, Marchandet L, Baud’huin M, Verrecchia F, Ory B, et al. Origin and therapies of osteosarcoma. Cancers (Basel). 2022;14:3503. doi: https://doi.org/10.3390/cancers14143503
2. Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. doi: https://doi.org/10.1016/j.ejca.2018.11.027
3. Stiller CA, Bielack SS, Jundt G, Steliarova-Foucher E. Bone tumours in European children and adolescents, 1978–1997. Report from the automated childhood cancer information system project. Eur J Cancer. 2006;42:2124–35. doi: https://doi.org/10.1016/j.ejca.2006.05.015
4. Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34. doi: https://doi.org/10.1002/ijc.24320
5. SEER. Surveillance, Epidemiology, and End Results (SEER) Program, incidence sub (1975–2017). SEER*Stat Database n.d.:9 [cited 2023 Jan 24] Registries. Available from: https://seer.cancer.gov/
6. Tsukamoto S, Errani C, Angelini A, Mavrogenis AF. Current treatment considerations for osteosarcoma metastatic at presentation. Orthopedics. 2020;43:e345–58. doi: https://doi.org/10.3928/01477447-20200721-05
7. Singh R, Valluri A, Didwania P, Lehrer EJ, Baliga S, Hiniker S, et al. Efficacy and safety of stereotactic body radiation therapy (SBRT) for pediatric malignancies: the LITE-SABR systematic review and meta-analysis. Adv Radiat Oncol. 2023;8:101123. doi: https://doi.org/10.1016/j.adro.2022.101123
8. Ahmed G, Zamzam M, Kamel A, Ahmed S, Salama A, Zaki I, et al. Effect of timing of pulmonary metastasis occurrence on the outcome of metastasectomy in osteosarcoma patients. J Pediatr Surg. 2019;54:775–9. doi: https://doi.org/10.1016/j.jpedsurg.2018.06.019
9. Silva JAM, Marchiori E, Macedo FCD, Silva PRGD, Amorim VB. Pulmonary metastasis of osteosarcoma: multiple presentations in a single patient. J Bras Pneumol. 2022;48:e20210478. doi: https://doi.org/10.36416/1806-3756/e20210478
10. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21:vii320–5. doi: https://doi.org/10.1093/annonc/mdq276
11. American Cancer Society. Survival rates for osteosarcoma. 2022 [cited 2023 Jan 28]. Available from: https://www.cancer.org/cancer/osteosarcoma/detection-diagnosis-staging/survival-rates.html
12. Tan G, Xu J, Yu Q, Yang Z, Zhang H. The safety and efficiency of photodynamic therapy for the treatment of osteosarcoma: a systematic review of in vitro experiment and animal model reports. Photodiagnosis Photodyn Ther. 2022;40:103093. doi: https://doi.org/10.1016/j.pdpdt.2022.103093
13. Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. 2014;20:4200–9. doi: https://doi.org/10.1158/1078-0432.CCR-13-2574
14. Ni M. [Update and interpretation of 2021 National Comprehensive Cancer Network (NCCN) “Clinical Practice Guidelines for Bone Tumors”]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021;35:1186–91. doi: https://doi.org/10.7507/1002-1892.202103073
15. Hattinger CM, Salaroglio IC, Fantoni L, Godel M, Casotti C, Kopecka J, et al. Strategies to overcome resistance to immune-based therapies in osteosarcoma. Int J Mol Sci. 2023;24:799. doi: https://doi.org/10.3390/ijms24010799
16. Evdokimova V, Gassmann H, Radvanyi L, Burdach SEG. Current state of immunotherapy and mechanisms of immune evasion in ewing sarcoma and osteosarcoma. Cancers (Basel). 2022;15:272. doi: https://doi.org/10.3390/cancers15010272
17. Li E, Zhong S, Ma G, Wang Q, Gao Y. MIR503HG overexpression inhibits the malignant behaviors of osteosarcoma cells by sponging miR-103a-3p. Crit Rev Eukaryot Gene Expr. 2022:33(3):1–11. doi: https://doi.org/10.1615/CritRevEukaryotGeneExpr.2022042373
18. Clapp B, Portela R, Sharma I, Nakanishi H, Marrero K, Schauer P, et al. Risk of non-hormonal cancer after bariatric surgery: meta-analysis of retrospective observational studies. Br J Surg. 2022;110:24–33. doi: https://doi.org/10.1093/bjs/znac343
19. Yeo SY, Bratke G, Grüll H. High intensity focused ultrasound for treatment of bone malignancies—20 years of history. Cancers (Basel). 2022;15:108. doi: https://doi.org/10.3390/cancers15010108
20. Guo S, Zhu X, Huang Z, Wei C, Yu J, Zhang L, et al. Genomic instability drives tumorigenesis and metastasis and its implications for cancer therapy. Biomed Pharmacother. 2023;157:114036. doi: https://doi.org/10.1016/j.biopha.2022.114036
21. Fazaeli H, Sheikholeslami A, Ghasemian F, Amini E, Sheykhhasan M. The emerging role of LncRNA FENDRR in multiple cancer: a review study. Curr Mol Med. 2022;22:606–29. doi: https://doi.org/10.2174/1566524022666220509122505
22. Shahpouri M, Adili-Aghdam MA, Mahmudi H, Jaymand M, Amoozgar Z, Akbari M, et al. Prospects for hypoxia-based drug delivery platforms for the elimination of advanced metastatic tumors: from 3D modeling to clinical concepts. J Control Release. 2023;353:1002–22. doi: https://doi.org/10.1016/j.jconrel.2022.12.009
23. Zheng Y, Chang X, Huang Y, He D. The application of antidepressant drugs in cancer treatment. Biomed Pharmacother. 2023;157:113985. doi: https://doi.org/10.1016/j.biopha.2022.113985
24. López-Otín C, Pietrocola F, Roiz-Valle D, Galluzzi L, Kroemer G. Meta-hallmarks of aging and cancer. Cell Metab. 2023;35:12–35. doi: https://doi.org/10.1016/j.cmet.2022.11.001
25. Khadembaschi D, Jafri M, Praveen P, Parmar S, Breik O. Does neoadjuvant chemotherapy provide a survival benefit in maxillofacial osteosarcoma: a systematic review and pooled analysis. Oral Oncol. 2022;135:106133. doi: https://doi.org/10.1016/j.oraloncology.2022.106133
26. Lim YY, Miskon A, Zaidi AMA, Megat Ahmad MMH, Abu Bakar M. Numerical simulation study on relationship between the fracture mechanisms and residual membrane stresses of metallic material. J Funct Biomater. 2022c;13:20. doi: https://doi.org/10.3390/jfb13010020
27. Lim YY, Miskon A, Zaidi AMA. CuZn complex used in electrical biosensors for drug delivery systems. Materials (Basel). 2022a;15:7672. doi: https://doi.org/10.3390/ma15217672
28. Lim YY, Miskon A, Zaidi AMA, Megat Ahmad MMH, Abu Bakar M. Structural characterization analyses of low brass filler biomaterial for hard tissue implanted scaffold applications. Materials (Basel). 2022d;15:1421. doi: https://doi.org/10.3390/ma15041421
29. Lim YY, Miskon A, Zaidi AMA. Structural strength analyses for low brass filler biomaterial with anti-trauma effects in articular cartilage scaffold design. Materials (Basel). 2022b;15:4446. doi: https://doi.org/10.3390/ma15134446
30. Fierro Pineda JC, Wedekind MF, Glod JW. Immunotherapy approaches for rare pediatric solid tumors: advances and future directions. Curr Opin Pediatr. 2023;35:63–74. doi: https://doi.org/10.1097/MOP.0000000000001206
31. Ke CH, Chiu YH, Huang KC, Lin CS. Exposure of immunogenic tumor antigens in surrendered immunity and the significance of autologous tumor cell-based vaccination in precision medicine. Int J Mol Sci. 2022;24:147. doi: https://doi.org/10.3390/ijms24010147
32. Lim YY, Zaidi AMA, Miskon A. Composing on-program triggers and on-demand stimuli into biosensor drug carriers in drug delivery systems for programmable arthritis therapy. Pharmaceuticals. 2022e;15:1330. doi: https://doi.org/10.3390/ph15111330
33. Evans HM, Long JA. Characteristic effects upon growth, oestrus and ovulation induced by the intraperitoneal administration of fresh anterior hypophyseal substance. Proc Natl Acad Sci. 1922;8:38–9. doi: https://doi.org/10.1073/pnas.8.3.38
34. Li CH, Liu WK. Human pituitary growth hormone. Experientia. 1964;20:169–78. doi: https://doi.org/10.1007/BF02135393
35. Jaffe N, Frei E, Watts H, Traggis D. High-dose methotrexate in osteogenic sarcoma: a 5-year experience. Cancer Treat Rep. 1978;62:259–64.
36. Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–30. doi: https://doi.org/10.1002/1097-0142(19820315)49:6<1221::AID-CNCR2820490625>3.0.CO;2-E
37. Zapf J, Schmid CH, Froesch ER. 1 Biological and immunological properties of insulin-like growth factors (IGF) I and II. Clin Endocrinol Metab. 1984;13:3–30. doi: https://doi.org/10.1016/S0300-595X(84)80006-7
38. Stashenko P, Dewhirst FE, Rooney ML, Desjardins LA, Heeley JD. Interleukin-1β is a potent inhibitor of bone formation in vitro. J Bone Miner Res. 2009;2:559–65. doi: https://doi.org/10.1002/jbmr.5650020612
39. Bengtsson BA, Edén S, Lönn L, Kvist H, Stokland A, Lindstedt G, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309–17. doi: https://doi.org/10.1210/jcem.76.2.8432773
40. Gentet JC, Brunat-Mentigny M, Demaille MC, Pein F, Avet-Loiseau H, Berger C, et al. Ifosfamide and etoposide in childhood osteosarcoma. A phase II study of the french society of paediatric oncology. Eur J Cancer. 1997;33:232–7. doi: https://doi.org/10.1016/S0959-8049(96)00439-X
41. McGary EC, Weber K, Mills L, Doucet M, Lewis V, Lev DC, et al. Inhibition of platelet-derived growth factor-mediated proliferation of osteosarcoma cells by the novel tyrosine kinase inhibitor STI571. Clin Cancer Res. 2002;8:3584–91.
42. Nardin A, Lefebvre M, Labroquere K, Faure O, Abastado J. Liposomal muramyl tripeptide phosphatidylethanolamine: targeting and activating macrophages for adjuvant treatment of osteosarcoma. Curr Cancer Drug Targets. 2006;6:123–33. doi: https://doi.org/10.2174/156800906776056473
43. Tang QL, Zhao ZQ, Li J, Liang Y, Yin JQ, Zou CY, et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011;311:113–21. doi: https://doi.org/10.1016/j.canlet.2011.07.016
44. Gordon EM, Chua-Alcala VS, Kim K, Baby R, Angel N, Quon D, et al. A phase I/II investigation of nivolumab and ABI-009 (nab -sirolimus) in advanced undifferentiated pleomorphic sarcoma (UPS), liposarcoma (LPS), chondrosarcoma (CS), osteosarcoma (OS), and Ewing sarcoma: preliminary efficacy and safety results. J Clin Oncol. 2019;37:11057. doi: https://doi.org/10.1200/JCO.2019.37.15_suppl.11057
45. Manji A, Samson Y, Deyell RJ, Johnston DL, Lewis VA, Zorzi AP, et al. Low-dose metronomic topotecan and pazopanib (TOPAZ) in children with relapsed or refractory solid tumors: a C17 Canadian phase I clinical trial. Cancers (Basel). 2022;14:2985. doi: https://doi.org/10.3390/cancers14122985
46. Gartrell J, Panetta JC, Baker SD, Chen YL, Hawkins DS, Ostrenga A, et al. The effects of pazopanib on doxorubicin pharmacokinetics in children and adults with non-rhabdomyosarcoma soft tissue sarcoma: a report from children’s oncology group and NRG oncology study ARST1321. Cancer Chemother Pharmacol. 2022;89:551–7. doi: https://doi.org/10.1007/s00280-022-04397-4
47. Szkandera J. Keeping up the ‘race pace’ in a patient with nonuterine leiomyosarcoma. Futur Oncol. 2022;18:12–6. doi: https://doi.org/10.2217/fon-2022-0636
48. Liu Z, Wang X, Wang J, Zhang P, Li C, Wang B, et al. Gemcitabine plus anlotinib is effective and safe compared to gemcitabine plus docetaxel in advanced soft tissue sarcoma. Front Oncol. 2022;12:922127. doi: https://doi.org/10.3389/fonc.2022.922127
49. Villaruz LC, Kelly K, Waqar SN, Davis EJ, Shapiro G, LoRusso P, et al. NCI 9938: phase I clinical trial of ATR inhibitor berzosertib (M6620, VX-970) in combination with irinotecan in patients with advanced solid tumors. J Clin Oncol. 2022;40:3012. doi: https://doi.org/10.1200/JCO.2022.40.16_suppl.3012
50. Plummer R, Dean E, Arkenau HT, Redfern C, Spira AI, Melear JM, et al. A phase 1b study evaluating the safety and preliminary efficacy of berzosertib in combination with gemcitabine in patients with advanced non-small cell lung cancer. Lung Cancer. 2022;163:19–26. doi: https://doi.org/10.1016/j.lungcan.2021.11.011
51. Brahmi M, Gautier J, Dufresne A, Marec-Berard P, Cropet C, Vizoso S, et al. REGOMAIN: a randomized, placebo-controlled, double-blinded, multicenter, comparative phase II study of the efficacy of regorafenib as maintenance treatment in patients (pts) with high-grade bone sarcomas (HGBS) at diagnosis or relapse and without complete. J Clin Oncol. 2022;40:TPS11585. doi: https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS11585
52. Vienot A, Vernerey D, Bouard A, Klajer E, Asgarov K, Kim S, et al. SO-20 stanniocalcin 1 (STC1) in patients with refractory colorectal cancer (CRC) treated with regorafenib: an exploratory analysis of the CORRECT trial. Ann Oncol. 2022;33:S365. doi: https://doi.org/10.1016/j.annonc.2022.04.419
53. Lee S, Cavaliere A, Gallezot JD, Keler T, Michelhaugh SK, Belitzky E, et al. [89Zr]ZrDFO-CR011 PET correlates with response to glycoprotein nonmetastatic melanoma B–targeted therapy in triple-negative breast cancer. Mol Cancer Ther. 2022;21:440–7. doi: https://doi.org/10.1158/1535-7163.MCT-21-0590
54. Lazaratos AM, Annis MG, Siegel PM. GPNMB: a potent inducer of immunosuppression in cancer. Oncogene. 2022;41:4573–90. doi: https://doi.org/10.1038/s41388-022-02443-2
55. Albarrán V, Villamayor ML, Chamorro J, Rosero DI, Pozas J, San Román M, et al. Receptor tyrosine kinase inhibitors for the treatment of recurrent and unresectable bone sarcomas. Int J Mol Sci. 2022;23:13784. doi: https://doi.org/10.3390/ijms232213784
56. Pearson AD, Gaspar N, Janeway K, Campbell-Hewson Q, Lawlor ER, Copland C, et al. Paediatric strategy forum for medicinal product development of multi-targeted kinase inhibitors in bone sarcomas. Eur J Cancer. 2022;173:71–90. doi: https://doi.org/10.1016/j.ejca.2022.06.008
57. Teo MYM, Fong JY, Lim WM, In LLA. Current advances and trends in KRAS targeted therapies for colorectal cancer. Mol Cancer Res. 2022;20:30–44. doi: https://doi.org/10.1158/1541-7786.MCR-21-0248
58. Al Shihabi A, Davarifar A, Nguyen HTL, Tavanaie N, Nelson SD, Yanagawa J, et al. Personalized chordoma organoids for drug discovery studies. Sci Adv. 2022;8:3674. doi: https://doi.org/10.1126/sciadv.abl3674
59. Cash T, Jonus HC, Tsvetkova M, Beumer JH, Lee JY, Henry C, et al. A phase I study of simvastatin in combination with topotecan and cyclophosphamide in pediatric patients with relapsed and/or refractory solid and CNS tumors. J Clin Oncol. 2020;38:10541. doi: https://doi.org/10.1200/JCO.2020.38.15_suppl.10541
60. Duarte JA, de Barros ALB, Leite EA. The potential use of simvastatin for cancer treatment: a review. Biomed Pharmacother. 2021;141:111858. doi: https://doi.org/10.1016/j.biopha.2021.111858
61. Beird HC, Bielack SS, Flanagan AM, Gill J, Heymann D, Janeway KA, et al. Osteosarcoma. Nat Rev Dis Prim. 2022;8:77. doi: https://doi.org/10.1038/s41572-022-00409-y
62. Pascual-Pasto G, Castillo-Ecija H, Unceta N, Aschero R, Resa-Pares C, Gómez-Caballero A, et al. SPARC-mediated long-term retention of nab-paclitaxel in pediatric sarcomas. J Control Release. 2022;342:81–92. doi: https://doi.org/10.1016/j.jconrel.2021.12.035
63. Digklia A, Kollar A, Kronig MN, Britschgi C, Rordorf T, Joerger M, et al. 1495P SAKK 57/16 nab-paclitaxel and gemcitabine in soft tissue sarcoma (NAPAGE): final results from the phase Ib/II trial with >2y median follow up. Ann Oncol. 2022;33:S1230. doi: https://doi.org/10.1016/j.annonc.2022.07.1598
64. Omar S, Albritton K, Heym K, Wang J, Ray A. Multimodal treatment of sarcomas linked to BCOR-CCNB3 fusion in pediatrics: a 3-patient case series. Clin Pediatr Hematol. 2022;29:60–4. doi: https://doi.org/10.15264/cpho.2022.29.2.60
65. Smida M, Ammar A, Fedhila F, Douira W, Sassi S. Periosteal preservation: a new technique in resection of bone high-grade malignant tumors in children—about eleven cases. World J Surg Oncol. 2022;20:312. doi: https://doi.org/10.1186/s12957-022-02749-1
66. Puppo M, Jaafar M, Diaz JJ, Marcel V, Clézardin P. MiRNAs and snoRNAs in bone metastasis: functional roles and clinical potential. Cancers (Basel). 2022;15:242. doi: https://doi.org/10.3390/cancers15010242
67. Sadeh M, Toledano H, Cohen IJ. A comprehensive review of neuropsychologic studies supports the concept that adequate folinic acid rescue prevents post methotrexate neurotoxicity. J Pediatr Hematol Oncol. 2023;45:1–11. doi: https://doi.org/10.1097/MPH.0000000000002604
68. Hainaut P, Pfeifer GP. Somatic TP53 mutations in the era of genome sequencing. Cold Spring Harb Perspect Med. 2016;6:a026179. doi: https://doi.org/10.1101/cshperspect.a026179
69. Li VD, Li KH, Li JT. TP53 mutations as potential prognostic markers for specific cancers: analysis of data from the cancer genome atlas and the international agency for research on cancer TP53 database. J Cancer Res Clin Oncol. 2019;145:625–36. doi: https://doi.org/10.1007/s00432-018-2817-z
70. Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, et al. TP53 variations in human cancers: new lessons from the IARC TP53 database and genomics data. Hum Mutat. 2016;37:865–76. doi: https://doi.org/10.1002/humu.23035
71. Synoradzki KJ, Bartnik E, Czarnecka AM, Fiedorowicz M, Firlej W, Brodziak A, et al. TP53 in biology and treatment of osteosarcoma. Cancers (Basel). 2021;13:4284. doi: https://doi.org/10.3390/cancers13174284
72. Lim YY, Zaidi AMA, Miskon A. Combining copper and zinc into a biosensor for anti-chemoresistance and achieving osteosarcoma therapeutic efficacy. Molecules. 2023;28:2920. doi: https://doi.org/10.3390/molecules28072920
73. Li S, Wang X. The potential roles of exosomal noncoding RNAs in osteosarcoma. J Cell Physiol. 2021;236:3354–65. doi: https://doi.org/10.1002/jcp.30101
74. Abhange K, Makler A, Wen Y, Ramnauth N, Mao W, Asghar W, et al. Small extracellular vesicles in cancer. Bioact Mater. 2021;6:3705–43. doi: https://doi.org/10.1016/j.bioactmat.2021.03.015
75. Kluszczynska K, Czyz M. Extracellular vesicles-based cell-cell communication in melanoma: new perspectives in diagnostics and therapy. Int J Mol Sci. 2023;24:965. doi: https://doi.org/10.3390/ijms24020965
76. Song H, Zhao J, Cheng J, Feng Z, Wang J, Momtazi-Borojeni AA, et al. Extracellular vesicles in chondrogenesis and Cartilage regeneration. J Cell Mol Med. 2021;25:4883–92. doi: https://doi.org/10.1111/jcmm.16290
77. Jothimani G, Pathak S, Dutta S, Duttaroy AK, Banerjee A. A comprehensive cancer-associated microRNA expression profiling and proteomic analysis of human umbilical cord mesenchymal stem cell-derived exosomes. Tissue Eng Regen Med. 2022;19:1013–31. doi: https://doi.org/10.1007/s13770-022-00450-8
78. Kwok ZH, Wang C, Jin Y. Extracellular vesicle transportation and uptake by recipient cells: a critical process to regulate human diseases. Processes. 2021;9:273. doi: https://doi.org/10.3390/pr9020273
79. Ross J, McIver Z, Lambert T, Piergentili C, Bird JE, Gallagher KJ, et al. Pore dynamics and asymmetric cargo loading in an encapsulin nanocompartment. Sci Adv. 2022;8:4461. doi: https://doi.org/10.1126/sciadv.abj4461
80. van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369–82. doi: https://doi.org/10.1038/s41580-022-00460-3
81. Xiang Y, Hu C, Wu G, Xu S, Li Y. Nanomaterial-based microfluidic systems for cancer biomarker detection: recent applications and future perspectives. TrAC Trends Anal Chem. 2023;158:116835. doi: https://doi.org/10.1016/j.trac.2022.116835
82. Liang Y, Fang D, Gao X, Deng X, Chen N, Wu J, et al. Circulating microRNAs as emerging regulators of COVID-19. Theranostics. 2023;13:125–47. doi: https://doi.org/10.7150/thno.78164
83. Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10:1799. doi: https://doi.org/10.3389/fimmu.2019.01799
84. Wei Z, Zheng D, Pi W, Qiu Y, Xia K, Guo W. Isoquercitrin restrains the proliferation and promotes apoptosis of human osteosarcoma cells by inhibiting the Wnt/β-catenin pathway. J Bone Oncol. 2023;38:100468. doi: https://doi.org/10.1016/j.jbo.2023.100468
85. Lu D, Liu R, Zhou Y, Zhang Z, Jiang X, Xu J, et al. FOXO3a-dependent up-regulation of HSP90 alleviates cisplatin-induced apoptosis by activating FUNDC1-mediated mitophagy in hypoxic osteosarcoma cells. Cell Signal. 2023;101:110500. doi: https://doi.org/10.1016/j.cellsig.2022.110500
86. Raimondi L, De Luca A, Gallo A, Costa V, Russelli G, Cuscino N, et al. Osteosarcoma cell-derived exosomes affect tumor microenvironment by specific packaging of microRNAs. Carcinogenesis. 2020;41:666–77. doi: https://doi.org/10.1093/carcin/bgz130
87. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–82. doi: https://doi.org/10.1038/s41577-019-0127-6
88. Wolf-Dennen K, Gordon N, Kleinerman ES. Exosomal communication by metastatic osteosarcoma cells modulates alveolar macrophages to an M2 tumor-promoting phenotype and inhibits tumoricidal functions. Oncoimmunology. 2020;9:1747677. doi: https://doi.org/10.1080/2162402X.2020.1747677
89. Lopes N, Vivier E, Narni-Mancinelli E. Natural killer cells and type 1 innate lymphoid cells in cancer. Semin Immunol. 2023;66:101709. doi: https://doi.org/10.1016/j.smim.2022.101709
90. Wu CC, Beird HC, Andrew Livingston J, Advani S, Mitra A, Cao S, et al. Immuno-genomic landscape of osteosarcoma. Nat Commun. 2020;11:1008. doi: https://doi.org/10.1038/s41467-020-14646-w
91. Verma A, Mathur R, Farooque A, Kaul V, Gupta S, Dwarakanath BS. T-Regulatory cells in tumor progression and therapy. Cancer Manag Res. 2019;11:10731–47. doi: https://doi.org/10.2147/CMAR.S228887
92. Ray SK, Mukherjee S. Altering landscape of cancer vaccines: unique platforms, research on therapeutic applications and recent patents. Recent Pat Anticancer Drug Discov. 2023;18:133–46. doi: https://doi.org/10.2174/1574892817666220414110335
93. Bayatipoor H, Mehdizadeh S, Jafarpour R, Shojaei Z, Pashangzadeh S, Motallebnezhad M. Role of NKT cells in cancer immunotherapy—from bench to bed. Med Oncol. 2022;40:29. doi: https://doi.org/10.1007/s12032-022-01888-5
94. Metelli A, Wu BX, Riesenberg B, Guglietta S, Huck JD, Mills C, et al. Thrombin contributes to cancer immune evasion via proteolysis of platelet-bound GARP to activate LTGF-β. Sci Transl Med. 2020;12:4860. doi: https://doi.org/10.1126/scitranslmed.aay4860
95. Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–43. doi: https://doi.org/10.1038/nature25492
96. Shima T, Shimoda M, Shigenobu T, Ohtsuka T, Nishimura T, Emoto K, et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci. 2020;111:727–38. doi: https://doi.org/10.1111/cas.14272
97. Wang X, Chen Z, Li B, Fan J, Xu W, Xiao J. Immunotherapy as a promising option for the treatment of advanced chordoma: a systemic review. Cancers (Basel). 2022;15:264. doi: https://doi.org/10.3390/cancers15010264
98. Huang T, Tan X, Huang H, Li YT, Liu BL, Liu KS, et al. Targeting cancer-associated fibroblast-secreted WNT2 restores dendritic cell-mediated antitumour immunity. Gut. 2022;71:333–44.
99. Yue P, Han B, Zhao Y. Focus on the molecular mechanisms of cisplatin resistance based on multi-omics approaches. Mol Omi. 2023;19:297–307. doi: https://doi.org/10.1039/D2MO00220E
100. Fan L, Qu Y, Tong W, Lin H, Xiao B. Application value of a selenium-hydroxyapatite nanodelivery system as osteosarcoma treatment. Mater Express. 2022;12:1033–41. doi: https://doi.org/10.1166/mex.2022.2256
101. Garcia-Ortega DY, Cabrera-Nieto SA, Caro-Sánchez HS, Cruz-Ramos M. An overview of resistance to chemotherapy in osteosarcoma and future perspectives. Cancer Drug Resist. 2022;5:762–93. doi: https://doi.org/10.20517/cdr.2022.18
102. Inui T, Nomoto R, Yokota J, Yamashita T, Okada K, Kishimoto W, et al. Establishment of MDR1-knockout human enteroids for pharmaceutical application. Drug Metab Pharmacokinet. 2023;48:100476. doi: https://doi.org/10.1016/j.dmpk.2022.100476
103. Cai JX, Liu JH, Wu JY, Li YJ, Qiu XH, Xu WJ, et al. Hybrid cell membrane-functionalized biomimetic nanoparticles for targeted therapy of osteosarcoma. Int J Nanomed. 2022;17:837–54. doi: https://doi.org/10.2147/IJN.S346685
104. Zhang XB, Zhang RH, Su X, Qi J, Hu YC, Shi JT, et al. Exosomes in osteosarcoma research and preclinical practice. Am J Transl Res. 2021;13:882–97.
105. Weinman MA, Ramsey SA, Leeper HJ, Brady JV, Schlueter A, Stanisheuski S, et al. Exosomal proteomic signatures correlate with drug resistance and carboplatin treatment outcome in a spontaneous model of canine osteosarcoma. Cancer Cell Int. 2021;21:245. doi: https://doi.org/10.1186/s12935-021-01943-7
106. Homayoonfal M, Asemi Z, Yousefi B. Potential anticancer properties and mechanisms of thymoquinone in osteosarcoma and bone metastasis. Cell Mol Biol Lett. 2022;27:21. doi: https://doi.org/10.1186/s11658-022-00320-0
107. Cheng B, Yu Q, Wang W. Intimate communications within the tumor microenvironment: stromal factors function as an orchestra. J Biomed Sci. 2023;30:1. doi: https://doi.org/10.1186/s12929-022-00894-z
108. Chaudhary A, Raza SS, Haque R. Transcriptional factors targeting in cancer stem cells for tumor modulation. Semin Cancer Biol. 2023;88:123–37. doi: https://doi.org/10.1016/j.semcancer.2022.12.010
109. Wu T, Yang W, Sun A, Wei Z, Lin Q. The role of CXC chemokines in cancer progression. Cancers (Basel). 2022;15:167. doi: https://doi.org/10.3390/cancers15010167
110. Wang Y, Chu Y, Li K, Zhang G, Guo Z, Wu X, et al. Exosomes secreted by adipose-derived mesenchymal stem cells foster metastasis and osteosarcoma proliferation by increasing COLGALT2 expression. Front Cell Dev Biol. 2020;8:353. doi: https://doi.org/10.3389/fcell.2020.00353
111. Marulanda J, Tauer JT, Boraschi-Diaz I, Bardai G, Rauch F. Effect of sclerostin inactivation in a mouse model of severe dominant osteogenesis imperfecta. Sci Rep. 2023;13:5010. doi: https://doi.org/10.1038/s41598-023-32221-3
112. Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020a;490:54–65. doi: https://doi.org/10.1016/j.canlet.2020.07.008
113. Tian B, Du X, Zheng S, Zhang Y. The role of tumor microenvironment in regulating the plasticity of osteosarcoma cells. Int J Mol Sci. 2022;23:16155. doi: https://doi.org/10.3390/ijms232416155
114. Han F, Pu P, Wang C, Ding X, Zhu Z, Xiang W, et al. Osteosarcoma cell-derived exosomal miR-1307 promotes tumorgenesis via targeting AGAP1. Biomed Res Int. 2021;2021:1–17. doi: https://doi.org/10.1155/2021/7358153
115. Tan L, Wang Y, Hu X, Min L. The roles of exosomes in metastasis of sarcoma: from biomarkers to therapeutic targets. Biomolecules. 2023;13:456. doi: https://doi.org/10.3390/biom13030456
116. Shiau JP, Chuang YT, Yen CY, Chang FR, Yang KH, Hou MF, et al. Modulation of AKT pathway-targeting miRNAs for cancer cell treatment with natural products. Int J Mol Sci. 2023;24:3688. doi: https://doi.org/10.3390/ijms24043688
117. Zhang H, Yu Y, Wang J, Han Y, Ren T, Huang Y, et al. Macrophages-derived exosomal lncRNA LIFR-AS1 promotes osteosarcoma cell progression via miR-29a/NFIA axis. Cancer Cell Int. 2021;21:192. doi: https://doi.org/10.1186/s12935-021-01893-0
118. Alfieri M, Meo L, Ragno P. Posttranscriptional regulation of the plasminogen activation system by non-coding RNA in cancer. Int J Mol Sci. 2023;24:962. doi: https://doi.org/10.3390/ijms24020962
119. Luo X, Li Y, Hua Z, Xue X, Wang X, Pang M, et al. Exosomes-mediated tumor metastasis through reshaping tumor microenvironment and distant niche. J Control Release. 2023;353:327–36. doi: https://doi.org/10.1016/j.jconrel.2022.11.050
120. Zhang W, Yan Y, Peng J, Thakur A, Bai N, Yang K, et al. Decoding roles of exosomal lncRNAs in tumor-immune regulation and therapeutic potential. Cancers (Basel). 2022;15:286. doi: https://doi.org/10.3390/cancers15010286
121. Li Q, Wang X, Jiang N, Xie X, Liu N, Liu J, et al. Exosome-transmitted linc00852 associated with receptor tyrosine kinase AXL dysregulates the proliferation and invasion of osteosarcoma. Cancer Med. 2020;9:6354–66. doi: https://doi.org/10.1002/cam4.3303
122. Zhang H, Du Y, Xin P, Man X. The LINC00852/miR-29a-3p/JARID2 axis regulates the proliferation and invasion of prostate cancer cell. BMC Cancer. 2022;22:1269. doi: https://doi.org/10.1186/s12885-022-10263-6
123. Ge X, Liu WW, Zhao W, Feng S, Duan A, Ji C, et al. Exosomal transfer of LCP1 promotes osteosarcoma cell tumorigenesis and metastasis by activating the JAK2/STAT3 signaling pathway. Mol Ther Nucleic Acids. 2020;21:900–15. doi: https://doi.org/10.1016/j.omtn.2020.07.025
124. Zhang H, Song J, Zhou X. Long noncoding RNA P53 upregulated regulator of P53 levels promotes osteogenic differentiation in osteoporosis progression through sponging miR-135a-5p. J Biomater Tissue Eng. 2022;12:2085–91. doi: https://doi.org/10.1166/jbt.2022.3125
125. Zhang K, Dong C, Chen M, Yang T, Wang X, Gao Y, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10:411–25. doi: https://doi.org/10.7150/thno.33482
126. Mei X, Zhang B, Zhao M, Lu Q. An update on epigenetic regulation in autoimmune diseases. J Transl Autoimmun. 2022;5:100176. doi: https://doi.org/10.1016/j.jtauto.2022.100176
127. Mazumdar A, Urdinez J, Boro A, Migliavacca J, Arlt MJE, Muff R, et al. Osteosarcoma-derived extracellular vesicles induce lung fibroblast reprogramming. Int J Mol Sci. 2020;21:5451. doi: https://doi.org/10.3390/ijms21155451
128. Tripathi R, Sinha NR, Kempuraj D, Balne PK, Landreneau JR, Juneja A, et al. Evaluation of CRISPR/Cas9 mediated TGIF gene editing to inhibit corneal fibrosis in vitro. Exp Eye Res. 2022;220:109113. doi: https://doi.org/10.1016/j.exer.2022.109113
129. Cheng Z, Wang L, Wu C, Huang L, Ruan Y, Xue W. Tumor-derived exosomes induced M2 macrophage polarization and promoted the metastasis of osteosarcoma cells through tim-3. Arch Med Res. 2021;52:200–10. doi: https://doi.org/10.1016/j.arcmed.2020.10.018
130. Gomes de Morais AL, Cerdá S, de Miguel M. New checkpoint inhibitors on the road: targeting TIM-3 in solid tumors. Curr Oncol Rep. 2022;24:651–8. doi: https://doi.org/10.1007/s11912-022-01218-y
131. Zhang Y, Liu Z, Yang X, Lu W, Chen Y, Lin Y, et al. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics. 2021;11:1473–92. doi: https://doi.org/10.7150/thno.51245
132. Wang Y, Han Y, Wang L, Zou M, Sun Y, Sun H, et al. Mycoplasma gallisepticum escapes the host immune response via gga-miR-365-3p/SOCS5/STATs axis. Vet Res. 2022;53:103. doi: https://doi.org/10.1186/s13567-022-01117-x
133. Zhang H, Wang J, Ren T, Huang Y, Yu Y, Chen C, et al. LncRNA CASC15 is upregulated in osteosarcoma plasma exosomes and CASC15 knockdown inhibits osteosarcoma progression by regulating miR-338-3p/RAB14 axis. Onco Targets Ther. 2020b;13:12055–66. doi: https://doi.org/10.2147/OTT.S282053
134. Chen C, Liu L. Silencing of lncRNA KLF3-AS1 represses cell growth in osteosarcoma via miR-338-3p/MEF2C axis. J Clin Lab Anal. 2022;36:e24698. doi: https://doi.org/10.1002/jcla.24698
135. Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235:4734–45. doi: https://doi.org/10.1002/jcp.29351
136. Wang Q, Zhou H, Zhu X, Jiang F, Yu Q, Zhang J, et al. miR-208 inhibits myocardial tissues apoptosis in mice with acute myocardial infarction by targeting inhibition of PDCD4. J Biochem Mol Toxicol. 2022;36:e23202. doi: https://doi.org/10.1002/jbt.23202
137. Huang Y, Liu W, He B, Wang L, Zhang F, Shu H, et al. Exosomes derived from bone marrow mesenchymal stem cells promote osteosarcoma development by activating oncogenic autophagy. J Bone Oncol. 2020;21:100280. doi: https://doi.org/10.1016/j.jbo.2020.100280
138. Yu Z, Tang H, Chen S, Xie Y, Shi L, Xia S, et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist Updat. 2022;67:100915. doi: https://doi.org/10.1016/j.drup.2022.100915
139. Zhong L, Liao D, Li J, Liu W, Wang J, Zeng C, et al. Rab22a-NeoF1 fusion protein promotes osteosarcoma lung metastasis through its secretion into exosomes. Signal Transduct Target Ther. 2021;6:59. doi: https://doi.org/10.1038/s41392-020-00414-1
140. Zeng C, Zhong L, Liu W, Zhang Y, Yu X, Wang X, et al. Targeting the lysosomal degradation of Rab22a-NeoF1 fusion protein for osteosarcoma lung metastasis. Adv Sci. 2022;10:2205483. doi: https://doi.org/10.1002/advs.202205483
141. Ye H, Hu X, Wen Y, Tu C, Hornicek F, Duan Z, et al. Exosomes in the tumor microenvironment of sarcoma: from biological functions to clinical applications. J Nanobiotechnol. 2022;20:403. doi: https://doi.org/10.1186/s12951-022-01609-0
142. Zhu T, Han J, Yang L, Cai Z, Sun W, Hua Y, et al. Immune microenvironment in osteosarcoma: components, therapeutic strategies and clinical applications. Front Immunol. 2022;13:907550. doi: https://doi.org/10.3389/fimmu.2022.907550
143. Liu Z, Zhou Z, Dang Q, Xu H, Lv J, Li H, et al. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics. 2022;12:6273–90. doi: https://doi.org/10.7150/thno.76854
144. Wang J, Zhang H, Sun X, Wang X, Ren T, Huang Y, et al. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J Nanobiotechnol. 2020;18:151. doi: https://doi.org/10.1186/s12951-020-00710-6
145. Chang SLY, Lee CW, Yang CY, Lin ZC, Peng KT, Liu SC, et al. IOX-1 suppresses metastasis of osteosarcoma by upregulating histone H3 lysine trimethylation. Biochem Pharmacol. 2023;210:115472. doi: https://doi.org/10.1016/j.bcp.2023.115472
146. Zhan X, He Q, Sheng J, Jiang X, Lin L, Huang Y, et al. USP12 positively regulates M-MDSC function to inhibit antitumour immunity through deubiquitinating and stabilizing p65. Immunology. 2022;167:544–57. doi: https://doi.org/10.1111/imm.13552
147. Zhang X, Jin X, Guan L, Lin X, Li X, Li Y. IgG4-Related disease with gastrointestinal involvement: case reports and literature review. Front Immunol. 2022;13. doi: https://doi.org/10.3389/fimmu.2022.816830
148. Chen Z, Yu H, Chen X, Chen W, Song W, Li Z. Mutual regulation between glycosylation and transforming growth factor-β isoforms signaling pathway. Int J Biol Macromol. 2023;236:123818. doi: https://doi.org/10.1016/j.ijbiomac.2023.123818
149. Chen SY, Mamai O, Akhurst RJ. TGFβ: signaling blockade for cancer immunotherapy. Annu Rev Cancer Biol. 2022;6:123–46. doi: https://doi.org/10.1146/annurev-cancerbio-070620-103554
150. Qin K, Yu M, Fan J, Wang H, Zhao P, Zhao G, et al. Canonical and noncanonical Wnt signaling: a comprehensive review of multilayered mediators, signaling mechanisms and crosstalk with major signaling pathways. Genes Dis. 2023. doi: https://doi.org/10.1016/j.gendis.2023.01.030
151. Duan Z, Lin X, Wang L, Zhen Q, Jiang Y, Chen C, et al. Specificity of TGF-β1 signal designated by LRRC33 and integrin αVβ8. Nat Commun. 2022;13:4988. doi: https://doi.org/10.1038/s41467-022-32655-9
152. Dodagatta-Marri E, Ma HY, Liang B, Li J, Meyer DS, Chen SY, et al. Integrin αvβ8 on T cells suppresses anti-tumor immunity in multiple models and is a promising target for tumor immunotherapy. Cell Rep. 2021;36:109309. doi: https://doi.org/10.1016/j.celrep.2021.109309
153. Guo D, Tong Y, Jiang X, Meng Y, Jiang H, Du L, et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metab. 2022;34:1312–24.e6. doi: https://doi.org/10.1016/j.cmet.2022.08.002
154. Zhang Y, Fu J, Liu S, Wang L, Qiu J, van Schaik EJ, et al. Coxiella burnetii inhibits host immunity by a protein phosphatase adapted from glycolysis. Proc Natl Acad Sci. 2022;119:e2110877119. doi: https://doi.org/10.1073/pnas.2110877119
155. Du L, He H, Xiao Z, Xiao H, An Y, Zhong H, et al. GSH-responsive metal–organic framework for intratumoral release of NO and IDO inhibitor to enhance antitumor immunotherapy. Small. 2022;18:2107732. doi:https://doi.org/10.1002/smll.202107732
156. Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110:102461. doi: https://doi.org/10.1016/j.ctrv.2022.102461
157. Gulley JL, Schlom J, Barcellos-Hoff MH, Wang X, Seoane J, Audhuy F, et al. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol Oncol. 2022;16:2117–34. doi: https://doi.org/10.1002/1878-0261.13146
158. Metropulos AE, Munshi HG, Principe DR. The difficulty in translating the preclinical success of combined TGFβ and immune checkpoint inhibition to clinical trial. EBioMedicine. 2022;86:104380. doi: https://doi.org/10.1016/j.ebiom.2022.104380
159. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39. doi: https://doi.org/10.1016/j.intimp.2018.06.001
160. Martin CJ, Datta A, Littlefield C, Kalra A, Chapron C, Wawersik S, et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci Transl Med. 2020;12:8456. doi: https://doi.org/10.1126/scitranslmed.aay8456
161. Chen C, Shi Q, Xu J, Ren T, Huang Y, Guo W. Current progress and open challenges for applying tyrosine kinase inhibitors in osteosarcoma. Cell Death Discov. 2022;8:488. doi: https://doi.org/10.1038/s41420-022-01252-6
162. Northcote-Smith J, Suntharalingam K. Targeting chemotherapy-resistant tumour sub-populations using inorganic chemistry: anti-cancer stem cell metal complexes. Curr Opin Chem Biol. 2023;72:102237. doi: https://doi.org/10.1016/j.cbpa.2022.102237
163. Costa AR, Duarte AC, Costa-Brito AR, Gonçalves I, Santos CRA. Bitter taste signaling in cancer. Life Sci. 2023;315:121363. doi: https://doi.org/10.1016/j.lfs.2022.121363
164. Aprile M, Costa V, Cimmino A, Calin GA. Emerging role of oncogenic long noncoding RNA as cancer biomarkers. Int J Cancer. 2023;152:822–34. doi: https://doi.org/10.1002/ijc.34282
165. Zhang Q, Liu N, Wang J, Liu Y, Wang K, Zhang J, et al. The recent advance of cell-penetrating and tumor-targeting peptides as drug delivery systems based on tumor microenvironment. Mol Pharm. 2023;20(2):789–809. doi: https://doi.org/10.1021/acs.molpharmaceut.2c00629
166. de Castro KC, Coco JC, dos Santos ÉM, Ataide JA, Martinez RM, do Nascimento MHM, et al. Pluronic® triblock copolymer-based nanoformulations for cancer therapy: a 10-year overview. J Control Release. 2023;353:802–22. doi: https://doi.org/10.1016/j.jconrel.2022.12.017
167. Wang H, Zhou X, Li C, Yan S, Feng C, He J, et al. The emerging role of pyroptosis in pediatric cancers: from mechanism to therapy. J Hematol Oncol. 2022;15:140. doi: https://doi.org/10.1186/s13045-022-01365-6
168. Marques da Costa ME, Marchais A, Gomez-Brouchet A, Job B, Assoun N, Daudigeos-Dubus E, et al. In-vitro and in-vivo establishment and characterization of bioluminescent orthotopic chemotherapy-resistant human osteosarcoma models in NSG mice. Cancers (Basel). 2019;11:997. doi: https://doi.org/10.3390/cancers11070997
169. Salem IM, Mostafa SM, Salama I, El-Sabbagh OI, Hegazy WAH, Ibrahim TS. Design, synthesis and antitumor evaluation of novel pyrazolo[3,4- d ]pyrimidines incorporating different amino acid conjugates as potential DHFR inhibitors. J Enzyme Inhib Med Chem. 2023;38:203–15. doi: https://doi.org/10.1080/14756366.2022.2142786
170. Yu W, Min D, Lin F, Zheng S, Tang L, He A, et al. SKA1 induces de novo MTX-resistance in osteosarcoma through inhibiting FPGS transcription. FEBS J. 2019;286:2399–414. doi: https://doi.org/10.1111/febs.14808
171. Zhang Y, Zhang C, Man X, Men Y, Ren X, Li X, et al. Functional characterization of the SiFPGS2 gene of foxtail millet in folate accumulation and root development. Plant Growth Regul. 2022. doi: https://doi.org/10.1007/s10725-022-00904-y
172. Xiao X, Wang W, Li Y, Yang D, Li X, Shen C, et al. HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res. 2018;37:201. doi: https://doi.org/10.1186/s13046-018-0880-6
173. Chen Y, Zhang K, Li Y, Guo R, Zhang K, Zhong G, et al. Oestrogen-related receptor alpha mediates chemotherapy resistance of osteosarcoma cells via regulation of ABCB1. J Cell Mol Med. 2019;23:2115–24. doi: https://doi.org/10.1111/jcmm.14123
174. Wang G, Cao L, Jiang Y, Zhang T, Wang H, Wang Z, et al. Anlotinib reverses multidrug resistance (MDR) in osteosarcoma by inhibiting P-glycoprotein (PGP1) function in vitro and in vivo. Front Pharmacol. 2022;12:798837. doi: https://doi.org/10.3389/fphar.2021.798837
175. He Q, Hao P, He G, Mai H, Liu W, Zhang W, et al. IGF2BP1-regulated expression of ERRα is involved in metabolic reprogramming of chemotherapy resistant osteosarcoma cells. J Transl Med. 2022;20:348. doi: https://doi.org/10.1186/s12967-022-03549-7
176. Nwabo Kamdje AH, Seke Etet PF, Kipanyula MJ, Vecchio L, Tagne Simo R, Njamnshi AK, et al. Insulin-like growth factor-1 signaling in the tumor microenvironment: carcinogenesis, cancer drug resistance, and therapeutic potential. Front Endocrinol (Lausanne). 2022;13:927390. doi: https://doi.org/10.3389/fendo.2022.927390
177. Celik B, Cicek K, Leal AF, Tomatsu S. Regulation of molecular targets in osteosarcoma treatment. Int J Mol Sci. 2022;23:12583. doi: https://doi.org/10.3390/ijms232012583
178. Li X, Liu YY, Zhang X, Shen J, Xu R, Liu YY, et al. Circular RNA hsa_circ_0000073 contributes to osteosarcoma cell proliferation, migration, invasion and methotrexate resistance by sponging miR-145-5p and miR-151-3p and upregulating NRAS. Aging (Albany NY). 2020;12:14157–73. doi: https://doi.org/10.18632/aging.103423
179. Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: a review of current and future strategies. Int J Mol Sci. 2020;21:6885. doi: https://doi.org/10.3390/ijms21186885
180. Wei W, Ji L, Duan W, Zhu J. Circular RNA circ_0081001 knockdown enhances methotrexate sensitivity in osteosarcoma cells by regulating miR-494-3p/TGM2 axis. J Orthop Surg Res. 2021;16:50. doi: https://doi.org/10.1186/s13018-020-02169-5
181. Liu S, Duan K, Zhang X, Cao X, Wang X, Meng F, et al. Circ_0081001 down-regulates miR-494-3p to enhance BACH1 expression and promotes osteosarcoma progression. Aging (Albany NY). 2021;13:17274–84. doi: https://doi.org/10.18632/aging.203207
182. Bai Y, Li Y, Bai J, Zhang Y. Hsa_circ_0004674 promotes osteosarcoma doxorubicin resistance by regulating the miR-342-3p/FBN1 axis. J Orthop Surg Res. 2021;16:510. doi: https://doi.org/10.1186/s13018-021-02631-y
183. Ma XL, Zhan TC, Hu JP, Zhang CL, Zhu KP. Doxorubicin-induced novel circRNA_0004674 facilitates osteosarcoma progression and chemoresistance by upregulating MCL1 through miR-142-5p. Cell Death Discov. 2021;7:309. doi: https://doi.org/10.1038/s41420-021-00694-8
184. Guan H, Xu H, Chen J, Wu W, Chen D, Chen Y, et al. Circ_0001721 enhances doxorubicin resistance and promotes tumorigenesis in osteosarcoma through miR-758/TCF4 axis. Cancer Cell Int. 2021;21:336. doi: https://doi.org/10.1186/s12935-021-02016-5
185. Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z, et al. CircMYO10 promotes osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to enhance the transcriptional activity of β-catenin/LEF1 complex via effects on chromatin remodeling. Mol Cancer. 2019;18:150. doi: https://doi.org/10.1186/s12943-019-1076-1
186. Wei W, Ji L, Duan W, Zhu J. CircSAMD4A contributes to cell doxorubicin resistance in osteosarcoma by regulating the miR-218-5p/KLF8 axis. Open Life Sci. 2020;15:848–59. doi: https://doi.org/10.1515/biol-2020-0079
187. Yanbin Z, Jing Z. CircSAMD4A accelerates cell proliferation of osteosarcoma by sponging miR-1244 and regulating MDM2 mRNA expression. Biochem Biophys Res Commun. 2019;516:102–11. doi: https://doi.org/10.1016/j.bbrc.2019.05.182
188. Ji Y, Liu J, Zhu W, Ji J. circ_0002060 enhances doxorubicin resistance in osteosarcoma by regulating the miR-198/ABCB1 axis. Cancer Biother Radiopharm. 2020:cbr.2020.4240. doi: https://doi.org/10.1089/cbr.2020.4240
189. Huang Y, Xie J, Li E. Comprehensive circular RNA profiling reveals circ_0002060 as a potential diagnostic biomarkers for osteoporosis. J Cell Biochem. 2019;120:15688–94. doi: https://doi.org/10.1002/jcb.28838
190. Xie C, Liang G, Xu Y, Lin E. Circular RNA hsa_circ_0003496 contributes to tumorigenesis and chemoresistance in osteosarcoma through targeting (microRNA) miR-370/Krüppel-like factor 12 axis. Cancer Manag Res. 2020;12:8229–40. doi: https://doi.org/10.2147/CMAR.S253969
191. Lin Z, Xie X, Lu S, Liu T. Noncoding RNAs in osteosarcoma: implications for drug resistance. Cancer Lett. 2021;504:91–103. doi: https://doi.org/10.1016/j.canlet.2021.02.007
192. Zhang Z, Zhou Q, Luo F, Zhou R, Xu J, Xiao J, et al. Circular RNA circ-CHI3L1.2 modulates cisplatin resistance of osteosarcoma cells via the miR-340-5p/LPAATβ axis. Hum Cell. 2021;34:1558–68. doi: https://doi.org/10.1007/s13577-021-00564-6
193. Song L, Zhou Z, Gan Y, Li P, Xu Y, Zhang Z, et al. Long noncoding RNA OIP5-AS1 causes cisplatin resistance in osteosarcoma through inducing the LPAATβ/PI3K/AKT/mTOR signaling pathway by sponging the miR-340-5p. J Cell Biochem. 2019;120:9656–66. doi: https://doi.org/10.1002/jcb.28244
194. Zhang J, Ma X, Zhou R, Zhou Y. TRPS1 and YAP1 regulate cell proliferation and drug resistance of osteosarcoma via competitively binding to the target of circTADA2A—miR-129-5p. Onco Targets Ther. 2020;13:12397–407. doi: https://doi.org/10.2147/OTT.S276953
195. Wu Y, Xie Z, Chen J, Chen J, Ni W, Ma Y, et al. Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol Cancer. 2019;18:73. doi: https://doi.org/10.1186/s12943-019-1007-1
196. Pan Y, Lin Y, Mi C. Cisplatin-resistant osteosarcoma cell-derived exosomes confer cisplatin resistance to recipient cells in an exosomal circ_103801-dependent manner. Cell Biol Int. 2021;45:858–68. doi: https://doi.org/10.1002/cbin.11532
197. Soghli Negin, Qujeq D, Yousefi T, Soghli Negar. The regulatory functions of circular RNAs in osteosarcoma. Genomics. 2020;112:2845–56. doi: https://doi.org/10.1016/j.ygeno.2020.03.024
198. Eaton BR, Schwarz R, Vatner R, Yeh B, Claude L, Indelicato DJ, et al. Osteosarcoma. Pediatr Blood Cancer. 2021;68. https://doi.org/10.1002/pbc.28352
199. Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, et al. Chronic stress promotes cancer development. Front Oncol. 2020;10:1492. doi: https://doi.org/10.3389/fonc.2020.01492
200. Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:767–85. doi: https://doi.org/10.1038/s41568-021-00395-5
201. Prater S, McKeon B. Osteosarcoma [Updated 2022 M 29]. Treasure Island, FL: StatPearls Publ; 2022 [cited 2023 Jan 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549868/
202. Czarnecka AM, Synoradzki K, Firlej W, Bartnik E, Sobczuk P, Fiedorowicz M, et al. Molecular biology of osteosarcoma. Cancers (Basel). 2020;12:2130. doi: https://doi.org/10.3390/cancers12082130
203. Brady SW, Ma X, Bahrami A, Satas G, Wu G, Newman S, et al. The clonal evolution of metastatic osteosarcoma as shaped by cisplatin treatment. Mol Cancer Res. 2019;17:895–906. doi: https://doi.org/10.1158/1541-7786.MCR-18-0620
204. Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362. doi: https://doi.org/10.1038/s41392-021-00670-9
205. Bleve A, Consonni FM, Porta C, Garlatti V, Sica A. Evolution and targeting of myeloid suppressor cells in cancer: a translational perspective. Cancers (Basel). 2022;14:510. doi: https://doi.org/10.3390/cancers14030510
206. Deng C, Xu Y, Fu J, Zhu X, Chen H, Xu H, et al. Reprograming the tumor immunologic microenvironment using neoadjuvant chemotherapy in osteosarcoma. Cancer Sci. 2020;111:1899–909. doi: https://doi.org/10.1111/cas.14398
207. Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T, et al. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol. 2019;31:187–98. doi: https://doi.org/10.1093/intimm/dxy079
208. Wright EM. SGLT2 and cancer. Pflügers Arch Eur J Physiol. 2020;472:1407–14. doi: https://doi.org/10.1007/s00424-020-02448-4
209. Barbosa AM, Martel F. Targeting glucose transporters for breast cancer therapy: the effect of natural and synthetic compounds. Cancers (Basel). 2020;12:154. doi: ttps://doi.org/10.3390/cancers12010154
210. Wu W, Zhang Z, Jing D, Huang X, Ren D, Shao Z, et al. SGLT2 inhibitor activates the STING/IRF3/IFN-β pathway and induces immune infiltration in osteosarcoma. Cell Death Dis. 2022;13:523. doi: https://doi.org/10.1038/s41419-022-04980-w