INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder triggered by defects in carbohydrate, fat, and protein metabolism and characterized by elevated blood glucose level (Rahimi et al., 2017). DM is a chronic disorder affecting almost any age group of people. Diabetes is the most common disease worldwide caused by the dysfunction of pancreatic β-cells and reduced sensitivity of insulin (Kumar et al., 2011). Insulin-dependent DM is characterized by a decreased insulin level. Pancreatic β-cells are affected which may be autoimmune or idiopathic with unknown specific causes. Non-insulin-dependent DM is characterized by development of insulin resistance (Marvibaigi et al., 2021). The World Health Organization (WHO) report suggests that the number of people living with diabetes in 1980 was 108 million, which then increased to 422 million in the year 2014 (Bansal et al., 2019). In the year 2012, because of diabetes, about 1.5 million people died globally. According to International Diabetes Federation (IDF), the number of people worldwide suffering from diabetes is expected to be increased to about 629 million by the year 2045 (Cho et al., 2018). The prevalence of diabetes in 1980 shifted from 4.7% to 8.5%, which was found to be double in three decades (Dra et al., 2019). Long-term chronic DM may result in to microvascular and macrovascular complication affecting kidney, eyes and neurons resulting in high morbidity and mortality (Oliveira et al., 2020;Sekiou et al., 2021). The treatment for diabetes currently focuses on the reduction of blood glucose levels by using insulin and oral hypoglycemic drugs. Individuals suffering from DM find it difficult to control using the present-day treatment option as they are associated with unwanted side effects and difficulty in regular administration (Mourya et al., 2017; Singh et al., 2020). Considering the limitations of the present therapy, the WHO recommended to find natural plants having the potential to treat DM. Many plants used historically proved effective and received significant attention as they are templates for the development of new drugs for diabetes. The plants form the basis for the traditional medicinal system since many years. Traditionally, more than 800 plants are considered effective for DM (Antora and Salleh, 2017; Cherbal et al., 2017). Considering its less toxicity and comparatively cost-effectiveness, plants are mostly preferred (Arumugam et al., 2013; Jacob and Narendhirakannan, 2019).
Ficus lacor Buch. Ham. is a large evergreen plant belonging to family Moraceae. It is commonly known as plaksha, pitana, karpari, or parkati (Khare, 2007; Sindhu and Arora, 2013b). Different parts of the plant like fruits, cork, and leaves are used traditionally for the treatment of many disorders. The common constituents reported in plant are long chain alcohols, sterols, beta sitosterol, and lanosterol (Ghimire et al., 2020). The plant is also found to contain triterpenoid, alpha and beta amyrin, and lupeol. Considering these active constituents present in the plant, it was used traditionally for treatment of leucorrhoea, typhoid fever, hay fever, snake bite, inflammation, arthritic, and skin problems (Ateeq et al., 2014; Sindhu and Arora, 2013a). The plant was found to have antioxidant property. The fresh ripe fruits of the plant were used traditionally for the treatment of diabetes (Khare, 2007). The other species of the plant were found to have scientifically proven antidiabetic activity (Deepa et al., 2018; Khan et al., 2013). Considering the traditional claim for use of fruits in diabetes, other species proved to have antidiabetic and antioxidant activities, which may prove its usefulness in the treatment of diabetes. Considering all the above claims, the present study was undertaken to evaluate the effect of F. lacor Buch. Ham. in streptozotocin (STZ)-induced diabetic rats.
MATERIALS AND METHODS
Chemicals
STZ used in the study was obtained from Sisco Research Laboratories, Mumbai, India. Standard drug glibenclamide was obtained from Yarrow Chem Products Ltd, Mumbai, India. Estimation of biochemical parameters was made using commercial kits from Coral Clinical Systems, Goa, India. Insulin estimation kit was obtained from Cusabio Biotech Ltd. The other experimental chemicals used were of analytical grade.
Plant materials
The fruits and cork of F. lacor Buch. Ham. were collected in the month of May from a locality in the village of Insuli, Sawantwadi, District Sindhudurg, Maharashtra, India. The plant specimen was authenticated and a voucher specimen was deposited with voucher number 12-B/226/2020 1 at the Department of Botany, Shri. Pancham Khemraj Mahavidyalay, Sawantwadi, Maharashtra, India.
Plant material processing and preparation of extracts
The fresh fruits and cork of F. lacor Buch. Ham. were washed with tap water and shade-dried at room temperature for 2 weeks. The dried fruits and cork were powdered by a mechanical grinder and stuffed with airproof pack. The powdered material (500 g) was extracted using Soxhlet apparatus for 72 hours with the help of ethanol as a solvent and the extract was dried in a rotary evaporator at 40°C for further study (Abubakar and Haque, 2020). The dried extracts were stored in an airtight container at 2°C–8°C for further use in the experiment.
Experimental animals
Adult female Wistar rats of (200–250 g) were housed at institutional animal houses at an appropriate temperature of 26°C ± 1°C and light controlled in a 12 hours light: 12 hours dark room with provision of food (Nutrivet Life Sciences, Pune, Maharashtra, India) and drinking water ad libitum. The study protocol was approved by the Institutional Animal Ethical Committee of Yashwantrao Bhonsale College of Pharmacy, Sawantwadi, India (Approval No: Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)/IAEC/2019-20/01). The facility provided and experiments were carried out as per “CPCSEA guidelines for animal house facility.”
Acute oral toxicity study
The acute toxicity study of fruit extract (FE) and cork extract (CE) of F. lacor Buch. Ham. was carried out as per the guidelines of the Organization for Economic Cooperation and Development (OECD 425 (Hazarika et al., 2019; Soliman, 2016). Acute toxicity study was carried out by selecting Wistar rats weighing between 200 and 250 g, and prior to dosing the animals were fasted overnight. Experimental animals were selected randomly and divided into three groups with one animal in each group. Doses were calculated based on their body weight; the first group received vehicle; second group received FE; and the third group received CE. Dosing was initiated at 1.75 mg/kg and continued up to 2,000 mg/kg as per the OECD 425 guidelines. The animals were observed continuously for the first 1 hour and intermittently for the next 4 hours and thereafter once every 24 hours for the next 14 days. During this period, observation was focused on mortality, behavioral and neurological parameters, and any other abnormality.
Experimental induction of diabetes
Rats weighing between 200 and 250 g were selected and diabetes was induced by the administration of STZ at the dose of 60 mg/kg freshly dissolved in cold 0.1 M citrate buffer, pH 4.5, via the intraperitoneal (i.p.) route (Azad and Sulaiman, 2020; Gebremeskel et al., 2020). 10% dextrose was thereafter administered orally to combat the immediate hypoglycemic death that could occur (Balaji et al., 2020). Diabetes was confirmed after 72 hours of STZ injection by measuring overnight fasting blood glucose level with the help of a glucometer (Glucoone, Dr. Morpen). Rats with a blood glucose level above 200 mg/dl were considered diabetic and used further in the experiment.
Experimental design
A total of 35 Wistar rats (5 normal and 30 STZ-diabetic) were used in study. The rats were divided randomly into seven groups with five animals in each group.
Group I: Normal control group (NC) received 2 ml/kg 0.5% Carboxymethyl Cellulose (CMC) in distilled water by oral route;
Group II: Diabetic control group (DC) received STZ 60 mg/kg (i.p.) and 2 ml/kg 0.5% CMC in distilled water by oral route;
Group III: Standard treatment group (STD) received STZ 60 mg/kg (i.p.) and glibenclamide 2 mg/kg by oral route;
Groups IV and V: FE treatment group (FE200 and FE400) received STZ 60 mg/kg (i.p.) and FE 200 and 400 mg/kg, respectively, by oral route;
Groups VI and VII: CE treatment group (CE200 and CE400) received STZ 60 mg/kg (i.p.) and CE 200 and 400 mg/kg, respectively, by oral route.
All the treatments were given via the oral route daily for a total of 21 days.
Body weight, food intake, water intake, and blood glucose level were measured on days 0, 7, 14, and 21 of the study (Ighodaro and Akinloye, 2017). About 2.5 ml of blood was collected from anesthetized animals through retro-orbital puncture method (Deutschlander et al., 2012) and serum was separated by centrifugation of blood at 5,000 rpm. On day 22, the animals were sacrificed by cervical dislocation and pancreas, liver, and kidney were dissected. The dissected organs were washed with ice cold saline and used for further experimental study.
Experimental scheme
The experimental scheme for the present research work is shown in Figure 1.
Oral glucose tolerance test (OGTT)
OGTT was carried out using acclimatized animals which were fasted for 24 hour along with water ad libitum before starting the experiment. The animals used were divided into seven groups containing five animals in each group. Group I served as NC, Group II was the diabetic control group, Group III was standard control receiving glibenclamide (2 mg/kg) by oral route, Groups IV–VII were plant treatment groups which received fruit and CE at 200 and 400 mg/kg dose by oral route. After withdrawing the initial (0 hour) blood samples and after 30 minutes of extract administration, the rats of all groups were orally administered 2 g/kg of glucose. Blood glucose level was checked after glucose loading at the interval of 30, 60, 90, and 120 minutes from tail vein by a glucometer.
Measurement of blood glucose, body weight, food intake, and water intake
Fasting blood glucose, body weight, food intake, and water intake were measured on days 0, 7, 14, and 21 of the study.
Estimation of serum insulin, insulin sensitivity index homeostasis model assessment of insulin resistance (HOMA-IR) and glycosylated hemoglobin
The fasting insulin level was estimated using commercial insulin estimation kit with the help of Elisa microplate reader. Glycosylated hemoglobin was estimated using commercial kit. The insulin sensitivity index using HOMA-IR calculated using the following formula:
Estimation of serum lipid profile markers
The serum lipid profile markers cholesterol, triglyceride, high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL), and total protein were estimated using commercial kits.
Estimation of kidney and liver serum markers
Fasting serum markers of kidney and liver like urea, uric acid, creatinine, aspartate aminotransferase (AST), and alanine transaminase (ALT) were estimated using commercial kits.
HISTOPATHOLOGICAL STUDIES
The dissected pancreas, livers, and kidneys were placed in 10% formalin with potassium phosphate buffer (0.1 M, pH 7.4). The tissue sample was then dehydrated using a series of graded alcohol and embedded in paraffin. Tissue section of about 3 μm was taken and stained with hematoxylin and eosin. The slides were observed under light microscope under ×100 magnification.
STATISTICAL ANALYSIS
The statistical analysis of the results was carried out by using software GraphPad prism version no: 5.0. The results obtained were expressed as mean ± standard error of mean (SEM). The statistical analysis of data was made by analysis of variance, followed by Dunnett’s test. A value of p < 0.05 was considered significant.
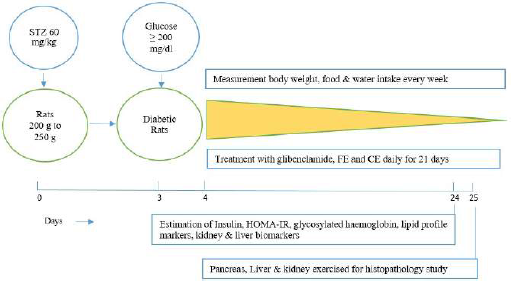 | Figure 1. Experimental scheme. [Click here to view] |
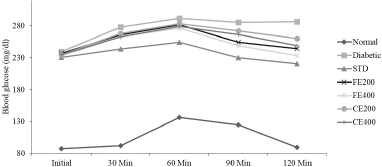 | Figure 2. Effect of fruit and CE of F. lacor Buch. Ham. on glucose tolerance test in STZ-induced diabetic rats. [Click here to view] |
RESULTS AND DISCUSSION
Considering the limitations of the currently available allopathic drugs, the present era is gaining more attention in the plant-oriented natural resources having antidiabetic activity. Considering the presence of antioxidant activity of F. lacor Buch. Ham. and its traditional claim for use in treatment of DM the present study was conducted.
ACUTE TOXICITY
The acute toxicity study was carried out for plant, plant products, and plant formulations to evaluate its safety using experimental animals. The toxicity study in animals determines safe dose for further study in animals which can also be extrapolated to human use. The anatomical and physiological parameters of experimental animals have very close relativeness with humans (Parasuraman, 2011). In acute toxicity study of fruit and CE of F. lacor Buch. Ham., mortality was absent at doses of 175, 550, and 2,000 mg/kg body weight. In the initial 1 hour and the following 24 hours of observation, the animals had not shown any adverse effect with respect to different parameters observed carefully. In the 14 days of observation, the animals had not shown any changes in skin, fur, eyes, mucous membranes, motor activity, respiratory, and central nervous system parameters. The absence of mortality and toxic symptoms during toxicity study indicates the lethal dose of both extracts might be above 2,000 mg/kg of body weight, indicating that the extracts were safe for further use.
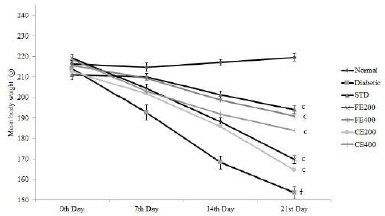 | Figure 3. Effect on body weight for fruit and CE of F. lacor Buch. Ham. Values are expressed as mean ± SEM (n = 5), ap ? 0.05, bp ? 0.01, cp ? 0.001, when compared with diabetic control group; dp ? 0.05, ep ? 0.01, fp ? 0.001, when compared with NC group. [Click here to view] |
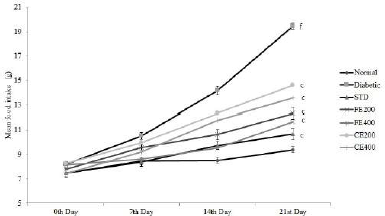 | Figure 4. Effect on food intake for fruit and CE of F. lacor Buch. Ham. Values are expressed as mean ± SEM (n = 5), ap < 0.05, bp < 0.01, cp < 0.001, when compared with diabetic control group; dp < 0.05, ep < 0.01, fp < 0.001, when compared with NC group. [Click here to view] |
INDUCTION OF DIABETES
In the present study, diabetes was induced by using single dose of STZ which was selectively taken by pancreatic beta-cells through glucose transporter causing destruction of pancreatic beta-cells. STZ-induced pancreatic damage is caused because of the alkylation of DNA through increased nitric oxide and nitrosourea, leading to hyperglycemia and reduced insulin and dyslipidemia (Hasan et al., 2018; Lenzen, 2008).
EFFECT OF THE EXTRACT IN OGTT
The OGTT revels that both FE and CE improve the handling of glucose by the diabetic rats similar to standard drug glibenclamide. The OGTT was impaired significantly in STZ-induced diabetic control rats when compared to NC rats. In the NC group, the glucose level increased steadily which reached maximum at 60 minutes, followed by a steady decrease which reached normal at 120 minutes. Whereas in the diabetic control group, the increased blood glucose level (after 60 minutes) remained steady even at 120 minutes, indicating impaired OGTT. In fruit and CE-treated groups, blood glucose increased at different time intervals and controlled significantly when compared to diabetic control group (Fig. 2).
EFFECT OF EXTRACT ON BODY WEIGHT, FOOD INTAKE, AND WATER INTAKE
Significant improvement in the body weight was observed after oral administration of glibenclamide, FE, and CE to diabetic rats. Food and water intake were increased in diabetic rats which can be reversed significantly by the oral administration of glibenclamide, FE, and CE. Hypoinsulinemia leads to an increase in catabolic reactions resulting in gluconeogenesis from lipids and proteins. This mechanism causes weight loss and activation of feeding center causing polyphagia (excessive eating). Hyperglycemia also results in the decreased reabsorption of glucose filtered by the kidneys; this increases the osmotic pressure of urine resulting in increased urine loss called polyuria. Excessive polyuria results in the loss of water and solutes resulting in polydipsia (excessive thirst) (Guo et al., 2018; Sharma et al., 2014) (Figs. 3–5).
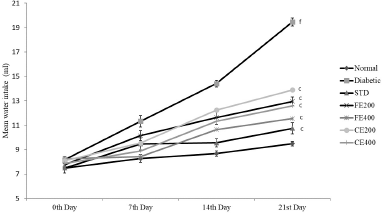 | Figure 5. Effect on water intake for fruit and CE of F. lacor Buch. Ham. Values are expressed as mean ± SEM (n = 5), ap < 0.05, bp < 0.01, cp < 0.001, when compared with diabetic control group; dp < 0.05, ep < 0.01, fp < 0.001, when compared with NC group. [Click here to view] |
EFFECT OF EXTRACT ON MEAN BLOOD GLUCOSE LEVEL
The administration of FE and CE significantly (p ? 0.001) reduces elevated blood glucose level in diabetic rats to a normal level in a dose-dependent manner. The hypoglycemic effect of both the extracts might be because of the increase in the release of insulin from pancreatic beta cells, increase in glucose uptake and reduced hepatic gluconeogenesis (Ezzat et al., 2014; Sharma et al., 2015). Hypoglycemic effect of FE and CE may be due to the restoration of pancreatic islet cell’s structure, resulting in improvement in its function to form and release insulin. The results also show that continuous treatment on a daily basis for 21 days significantly reduces blood glucose level in diabetic rats (Fig. 6).
EFFECT OF EXTRACT ON SERUM INSULIN, INSULIN SENSITIVITY INDEX (HOMA-IR), AND GLYCOSYLATED HEMOGLOBIN
STZ-induced type 1 DM is characterized by destruction of pancreatic beta cells resulting in reduced insulin level. Treatment with glibelclamide, FE, and CE restores structure of pancreatic beta-cells attributed to increased insulin level. Insulin resistance can be determined by mathematical model HOMA-IR based on blood glucose and insulin level. Increased HOMA-IR index indicates increased insulin resistance (Patil et al., 2014). Reduced insulin level in diabetic rat and hyperglycemia (type 1 DM) was reversed by treatment with glibenclamide, FE, and CE. The insulin sensitivity was found to increase (reduced HOMA-IR) significantly (p ? 0.05) by fruit and CE of F. lacor Buch. Ham. in dose-dependent manner, indicating FE is more effective compare to CE. Hyperglycemia as result of STZ-induced beta cell damage leads to oxidation of excessive glucose and which forms reactive oxygen species and glycation of protein result in increased HbA1c level in diabetic rats. The elevated HbA1c was reduced significantly by FE and CE may be due to its antioxidant activity which prevents glycation of protein. Glycosylated hemoglobin level was found to reduce significantly (p ? 0.01, p ? 0.001) in fruit and CE treated rats when compared with diabetic control group (Fig. 7).
EFFECT OF EXTRACT ON SERUM LIPID PROFILE MARKERS
In long-term DM, one of the secondary complications is dyslipidemia. This might be a result of reduced insulin level which activates the lipid metabolizing enzyme lipase. Increase in lipase level in diabetes attributes to increase in level of cholesterol, triglyceride, LDL, and VLDL and reduces HDL level as result of increased deposited lipid metabolism (Sztalryd and Kraemer, 1995). The elevated fatty acids have the effect on endothelial cell lining causing damage and responsible for micro and macro vascular complications (Ramachandran et al., 2012). The diabetic complications are the result of dyslipidemia through which reactive oxygen species may be formed. FE and CE significantly normalizes different lipid parameters like cholesterol, triglyceride, HDL, LDL, and VLDL (Table 1).
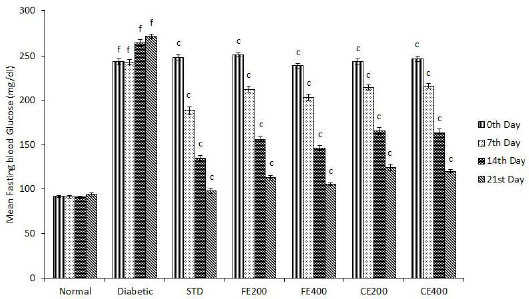 | Figure 6. Effect of fruit and CE of F. lacor Buch. Ham. on mean fasting blood glucose level in STZ-induced diabetic rats. Values are expressed as mean ± SEM (n = 5), ap ? 0.05, bp ? 0.01, cp ? 0.001, when compared with diabetic control group; dp ? 0.05, ep ? 0.01, fp ? 0.001, when compared with NC group. [Click here to view] |
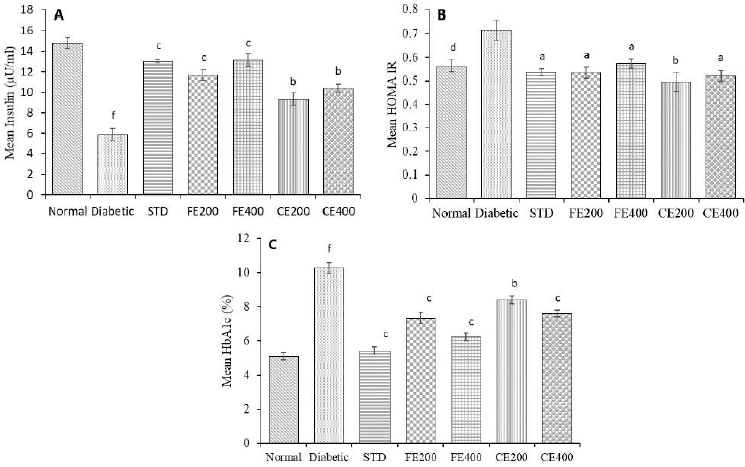 | Figure 7. Effect of fruit and CE of F. lacor Buch. Ham. on serum A. insulin, B. insulin sensitivity index (HOMA-IR), and C. glycosylated hemoglobin in STZ-induced diabetic rats. Values are expressed as mean ± SEM (n = 5), ap ? 0.05, bp ? 0.01, cp ? 0.001, when compared with diabetic control group; dp ? 0.05, ep ? 0.01, fp ? 0.001, when compared with NC group. [Click here to view] |
EFFECT OF EXTRACT ON KIDNEY AND LIVER SERUM BIOMARKERS
In present study, FE and CE treatment reduced significantly elevated serum urea, uric acid, and creatinine level in diabetic rats. The elevated urea is the result of improved gluconeogenesis due to insulin deficiency in rats produced by STZ. The reduced uptake of glucose by liver and other tissues due to insufficient insulin activates gluconeogenesis from proteins resulting in formation of excess amino acids which get converted to urea. Creatinine (metabolite of muscle) and uric acid (metabolite of purines) are a result of normal metabolic process which is eliminated by the kidney (Poongothai et al., 2011; Ramachandran et al., 2011). Elevated level of creatinine, uric acid, and urea indicates impaired function of the kidney as they are eliminated easily by kidney. The FE and CE administration to diabetic animals daily for 21 days protected kidney diminishing which result in to reduction of creatinine, uric acid, and urea level.
In liver, AST and ALT are the major transaminases enzymes which are released in to blood stream when there is liver toxicity. The chronic diabetic rat’s hepatic cells’ damage was confirmed by histopathological evaluation. In diabetic rats, elevated level of AST and ALT indicates the impairment of liver due to DM (Daisy and Kani, 2013). Treatment of diabetic rats with glibenclamide, FE, and CE daily for 21 days protected liver diminishing resulting in reduced levels of AST and ALT (Table 2).
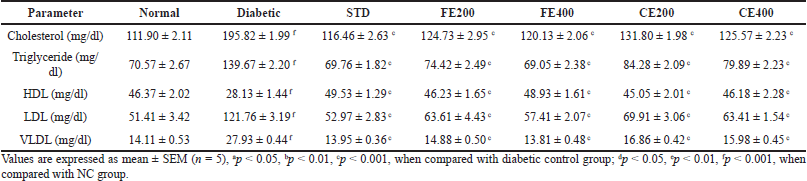 | Table 1. Effect of fruit and CE of F. lacor Buch. Ham. on serum lipid profile makers in STZ-induced diabetic rats. [Click here to view] |
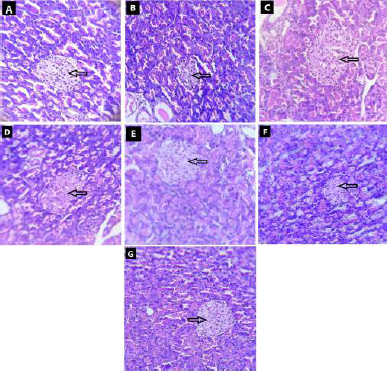 | Figure 8. Effect of fruit and CE of F. lacor Buch. Ham. on histopathology of pancreas by hematoxylin and eosin staining. A. NC, B. diabetic control, C. STD treatment group, D. FE200 treatment group, E. FE400 treatment group, F. CE200 treatment group, and G. CE400 treatment group. [Click here to view] |
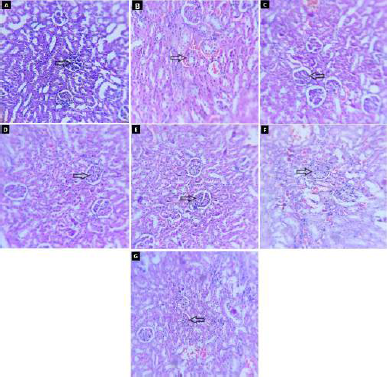 | Figure 9. Effect of fruit and CE of F. lacor Buch. Ham. on histopathology of kidney by hematoxylin and eosin staining. A. NC, B. diabetic control, C. STD treatment group, D. FE200 treatment group, E. FE400 treatment group, F. CE200 treatment group, and G. CE400 treatment group. [Click here to view] |
HISTOPATHOLOGY STUDY
Photomicrographs of the pancreas of diabetic control rat show destruction of beta-cells when compared with NC rats in which pancreas showed intact structure of pancreatic beta-cells. In NC rats, many round shaped islet cells (indicated by arrow) were present in the surrounding cytoplasm, the nucleus of islet cells was found to stained less compare to surrounding acinar cell’s nucleus. The diabetic rat showed shrunken and damages beta-cells of islets of Langerhans. Treatment of diabetic rats with glibenclamide, FE, and CE showed the restoration of normal structure of pancreatic beta-cells. The shrinkage and damage caused in diabetic rat were prevented in treatment groups with very small damaged islet cells observed (Fig. 8).
The histopathology of kidneys reveals that NC rats have normal glomeruli with basement membrane and tubular structure. Diabetic control rat showed morphological changes in glomeruli matrix and increased thickness of basement membrane (indicated by arrow) along with tubular collapse. In glibenclamide, FE and CE treated groups’ structure was restored more or less like NC rat (Fig. 9).
In NC rats, the intact structure of liver was observed with central vein radiating the hepatocyte which forms the lobules of the liver (indicated by arrow). The narrow blood sinusoids were also observed between radiating hepatocytes. The diabetic rat showed necrosis of the hepatocytes near central vein which was congested and having surrounding inflammatory cells. Treatment of diabetic rats with glibenclamide, FE, and CE showed the restoration of normal structure of liver. Necrosis of hepatocytes was prevented in all treatment groups where the structure of liver was found to be like NC rats (Fig. 10).
CONCLUSION
In present study, the traditional use of F. lacor Buch. Ham. can be justified for antidiabetic activity. The extracts showed that the significant hypoglycemia effect may be due to its capability to restore the pancreatic beta-cell structure resulting in the increase in blood insulin level. Oral administration of both extracts was found to improve body weight, reduce food intake, and water intake in diabetic rats. FE and CE treatment showed significant improvement in lipid profile in diabetic rats which reduces the probable complications associated with dyslipidemia. The kidney and liver markers were significantly normalized, which signifies a protective effect of the extract in both organs. Comparatively, FE was found to be more effective than CE in overall activity. This proves that F. lacor Buch. Ham. may be one of the alternatives for management of diabetes and related complications. Furthermore, a mechanism-based comprehensive study of the extract for antidiabetic activity needs to be carried out.
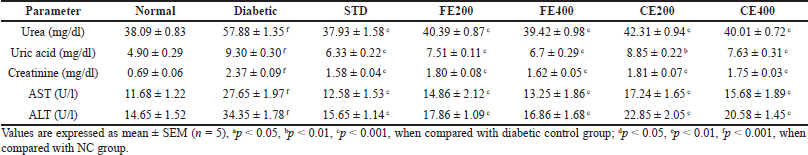 | Table 2. Effect of fruit and CE of F. lacor Buch. Ham. on kidney and liver serum markers in STZ-induced diabetic rats. [Click here to view] |
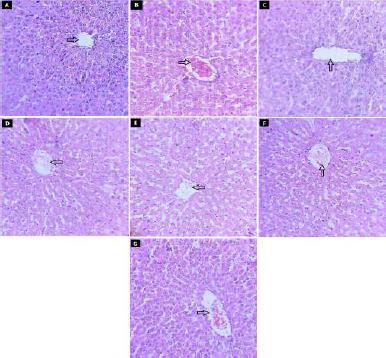 | Figure 10. Effect of fruit and CE of F. lacor Buch. Ham. on histopathology of liver by hematoxylin and eosin staining. A. NC, B. diabetic control, C. STD treatment group, D. FE200 treatment group, E. FE400 treatment group, F. CE200 treatment group, and G. CE400 treatment group. [Click here to view] |
AUTHORS’ CONTRIBUTIONS
Mule V. S. carried out all the above research activities. Mule V. S. and Naikwade N. S. participated in the concept, design, acquisition of data, analysis and interpretation of data, drafting, and revision of article. All authors read and approved the final manuscript for publication. Naikwade N. S. supervised all research work.
ACKNOWLEDGMENT
The authors are thankful to the principal, Yashwantrao Bhonsale College of Pharmacy, Sawantwadi, Maharashtra, India, for providing the necessary facilities.
CONFLICT OF INTEREST
The authors report no conflict of interest in this work.
FUNDING
The authors are thankful to the University of Mumbai, Mumbai, for funding this project (Grant Number: 201920/762/499).
REFERENCES
Abubakar AR, Haque M. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J Pharm Bioall Sci, 2020; 12:1–10. CrossRef
Antora RA, Salleh RM. Antihyperglycemic effect of Ocimum plants: a short review. Asian Pac J Trop Biomed, 2017; 7(8):755–9. CrossRef
Arumugam G, Manjula P, Paari N. A review: anti diabetic medicinal plants used for diabetes mellitus. J Acute Dis, 2013; 196–200. CrossRef
Ateeq A, Kumar MS, Ankit S, Kumar SA. Pharmacognostical evaluation of the fruit of plaksha—Ficus lacor Buch. Ham. Global J Res Med Plants Indigen Med, 2014; 3(4):165–74.
Azad AK, Sulaiman WM. Antidiabetic effects of P. macrocarpa ethanolic fruit extract in streptozotocin induced diabetic rats. Future J Pharm Sci, 2020; 6(57):1–12. CrossRef
Balaji P, Madhanraj R, Rameshkumar K, Veeramanikandan V, Eyini M, Arun A, Thulasinathan B, Farraj DA, Elshikh MS, Alokda AM, Mahmoud AH, Tack DC, Kim HJ. Evaluation of antidiabetic activity of Pleurotus pulmonarius against streptozotocin-nicotinamide induced diabetic Wistar albino rats. Saudi J Biol Sci, 2020; 27:913–24. CrossRef
Bansal K, Reddy KR, Trugunayat A. Antihyperglycemic study of Eladi Churna in streptozotocin induced diabetic rats. Indian J Tradit Knowl, 2019; 18(1):34–40.
Cherbal A, Kebieche M, Yilmaz EM, Aydogmus Z, Benzaouia L, Benguessoum M, Benkedidah M, Madani K. Antidiabetic and hypolipidemic activities of Algerian pistachia lentiscus L. leaves extract in alloxan induced diabetic rats. South Afr J Bot, 2017; 108:157–62. CrossRef
Cho NH, Shaw JE, Karuranga S, Huang Y, Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract, 2018; 138:271–81. CrossRef
Daisy P, Kani FG. Hypolipidemic and hepatoprotective effects of Cassia auriculata linn bark extracts on streptozotocin induced diabetics in male Wistar albino rats. Asian J Pharm Clin Res, 2013; 6(2):43–8.
Deepa P, Sowndhararajan K, Kim S, Park SJ. A role of Ficus species in the management of diabetes mellitus: a review. J Ethnopharmacol, 2018; 215:210–32. CrossRef
Deutschlander MS, Lall N, Van de Vanter M, Dewanjee S. The hypoglycemic activity of Euclea undulate Thunb. Var. Myrtina (Ebenaceae) root bark evaluated in a streptozotocin-nicotinamide induced type 2 diabetes rat model. South Afr J Bot, 2012; 80:9–12. CrossRef
Dra LA, Sellami A, Rais H, Aziz F, Aghraz A, Bekkouche K, Markouk M, Larhsini M. Antidiabetic potential of Caralluma europaea against alloxan induced diabetes in mice. Saudi J Biol Sci, 2019; 26:1171–8. CrossRef
Ezzat SM, Sattar EA, Harraz FM, Ghareib SA. Antihyperglycemic and antihyperlipidemic effects of the methanol extracts of Cleome ramosissima Parl., Barleria bispinosa (Forssk.) Vahl. and Tribulus macropterus Boiss. Bull Fac Pharm Cairo Univers, 2014; 52:1–7. CrossRef
Gebremeskel L, Tuem KB, Teklu T. Evaluation of antidiabetic effect of ethanolic leaves extract of Becium grandiflorum Lam. (Lamiaceae) in streptozotocin-induced diabetic mice. Diabetes Metab Syndr Obes Targets Ther, 2020; 13:1481–9. CrossRef
Ghimire P, Pandey S, Shrestha AC. A comprehensive review on phytochemical, pharmacognostical properties and pharmacological activities of Ficus lacor L. (Moraceae). Int J Herb Med, 2020; 8(5):96–102.
Guo X, Wang Y, Wank K, Ji BP, Zhou F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J Zhejiang Univ Sci B, 2018; 19(7):559–69. CrossRef
Hasan M, Ahmed QU, Soad SZ, Tunna TS. Animal models and natural products to investigate in vivo and in vitro antidiabetic activity. Biomed Pharmacother, 2018; 101:833–41. CrossRef
Hazarika I, Geetha KM, Sundari PS, Madhu D. Acute oral toxicity evaluation of extracts of Hydrocotyle sibthorpioides in Wistar albino rats as per OECD 425 TG. Toxicol Rep, 2019; 6:321–8. CrossRef
Ighodaro OM, Akinloye OA. Anti-diabetic potential of Sapium ellipticum (Hochst) Pax leaf extract in streptozotocin (STZ) induced diabetic Wistar rats. BMC Complement Altern Med, 2017; 17:525–32. CrossRef
Jacob B, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. 3Biotech, 2019; 9(4):1–17. CrossRef
Khan KY, Khan MA, Ahmad M, Hussain I, Mazari P, Fazak H, Ali B, Khan IZ. Hypoglycaemic potential of genus Ficus L.: a review on ten years of plant-based medicine used to cure diabetes (2000–2010). J Appl Pharm Sci, 2013; 1(6):223–7.
Khare CP. 2007. Indian medicinal plants an illustrated dictionary. Springer (India) Private Limited, New Delhi, India.
Kumar D, Kumar S, Kohli S, Arya R, Gupta J. Antidiabetic activity of methanolic bark extract of Albizia odoratissima Benth. in alloxan induced diabetic albino mice. Asian Pac J Trop Med, 2011; 4:900–3. CrossRef
Lenzen S. The mechanism of alloxan and streptozotocin induced diabetes. Diabetologia, 2008; 51:216–26. CrossRef
Marvibaigi M, Hosseini SM, Amini N. Launaea acanthodes (Boiss) O. Kuntze mediates hepatic glucose metabolism and ameliorates impaired pancreatic function in streptozotocin induced diabetic rats. J Ethnopharmacol, 2021; 268:113577. CrossRef
Mourya P, Shukla A, Rai G, Lodhi S. Hypoglycemic and hypolipidemic effects of ethanolic and aqueous extracts from Ziziphus oenoplia (L) Mill on alloxan induced diabetic rats. Beni-Suef Univers J Basic Appl Sci, 2017; 6:1–9. CrossRef
Oliveira ML, Cunha AL, Geraldo LW, Caetano CF, Caldeira CD. Silymarin attenuates hepatic and pancreatic redox imbalance independent of glycemic regulation in the alloxan induced diabetic rat model. Biomed Environ Sci, 2020; 33(9):690–700.
Parasuraman S. Toxicological screening. J Pharmacol Pharmacother, 2011; 2(2):74–9. CrossRef
Patil MA, Suryanarayana P, Putcha UK, Srinivas M, Reddy GB. Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid Med Cell Longevity, 2014; 2014:1–10. CrossRef
Poongothai K, Ponmurugun P, Ahmed KS, Kumar BS, Sherift SA. Antihyperglycemic and antioxidant effects of Solanum xanthocarpum leaves (field grown & in vitro raised) extracts on alloxan induced diabetic rats. Asian Pac J Trop Med, 2011; 778–85. CrossRef
Rahimi M, Heidarian E, Kheiri S, Rafieian M. Effect of hydroalcoholic Allium ampeloprasum extract on oxidative stress, diabetes mellitus and dyslipidemia in alloxan-induced diabetic rats. Biomed Pharmacother, 2017; 86:363–7. CrossRef
Ramachandran S, Rajasekaran A, Manisenthilkumar KT. Investigation of hypoglycemic, hypolipidemic and antioxidant activities of aqueous extract of Terminalia paniculata bark in diabetic rats. Asian Pac J Trop Biomed, 2012; 2(4):262–8. CrossRef
Ramachandran S, Rajashekaran A, Kumar KR. Antidiabetic, antilipidemic and antioxidant potential of methanol, extract of Tectina grandis flowers in streptozotocin induced diabetic rats. Asian Pac J Trop Med, 2011; 4:624–31. CrossRef
Sekiou O, Boumendjel M, Taibi F, Tichati L, Boumendjel A, Messarah M. Nephroprotective effect of Artemisia herba alba aqueous extract in alloxan induced diabetic rats. J Tradit Complement Med, 2021; 11:53–6. CrossRef
Sharma R, Amin H, Prajapati PK. Antidiabetic claims of Tinospora cordifolia (Willd.) Miers: critical appraisal and role in therapy. Asian Pac J Trop Biomed, 2015; 5(1):68–78. CrossRef
Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC. Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in streptozotocin induced diabetes in albino mice. Bull Fac Pharm Cairo Univers, 2014; 52:211–7. CrossRef
Sindhu RK, Arora S. Phytochemical and pharmacognostical investigations on arial roots of Ficus lacor Buch. Ham. Int J Phytomed, 2013a; 5:267–77.
Sindhu RK, Arora S. Therapeutic effect of Ficus lacor arial roots of various fractions on adjuvant induced arthritic rats. USRN Pharmacol, 2013b; 2013:1–8 CrossRef
Singh H, Singh R, kaur S, Arora R, Mannan R, Buttar HS, Arora S, Singh B. Protective role of Phyllanthus fraternus in alloxan induced diabetes in rats. J Ayurveda Integr Med, 2020; 11:391–8. CrossRef
Soliman AM. Potential impact of Paracentrotus lividus extract on diabetic rat models induced by high fat diet/streptozotocin. J Basic Appl Zool, 2016; 77:8–20. CrossRef
Sztalryd C, Kraemer FB. Regulation of hormone-sensitive lipase in streptozotocin-induced diabetic rats. Metabolism, 1995; 44(11):1391–6. CrossRef