INTRODUCTION
There are various types of mushrooms possessing high medicinal value for humans and one of them is known as Cordyceps. Cordyceps are classified as macrofungi due to their parasitic characteristic of insect larvae and pupae. The genus Cordyceps is an important group of medicinal fungi, a member of Ascomycota, Pyrenomycetes, Hypocreales, and Clavicipitaceae (Ng and Wang, 2005; Shrestha and Sung, 2005; Wong et al., 2007; Xiao et al., 2013; Yu et al., 2006). Cordyceps militaris, used in traditional Chinese remedies, contain several forms of phytochemicals such as cordycepin, cordycepic acid, sterols, nucleosides, and polysaccharides, which were proven to be beneficial for medicinal purposes (Tuli et al., 2013; Yue et al., 2013). Cordyceps militaris has been documented to enhance several pharmacological properties, including antioxidant, immunomodulatory, anti-inflammatory, antimicrobial, and antitumor properties. However, the potency of each property is significantly different based on the specific extract ingredients (Lee et al., 2006; Park et al., 2005; Yue et al., 2013; Zhou et al., 2009).
The abundance of polyphenolic content found in this mushroom might have played an important role in the observed antioxidant capacity due to the ability of single electron transfer to scavenge free radical atoms (Joshi and Sagar, 2014; Palacios et al., 2011). Cordyceps militaris extract (CME) also was reported to have a potential impact on cytotoxic against numerous human cancer cells, such as lung carcinoma cells (Lim et al., 2009; Park et al., 2009). Another research recorded that extraction of Ergosterol peroxide compound from C. militaris has a high potential effect on the Korean gastric cancer cell line (in vitro) (Kim et al., 2011). The water extract of phytochemicals from C. militaris showed positive activity on the human cancer cell lines such as adenocarcinoma, colorectal adenocarcinoma, and hepatocellular carcinoma, respectively. Cordycepin was one of the active compounds in CME that was thought to have an impact on human cancer cell lines (Lim et al., 2004). As far as we are aware, the antioxidant and anticancer properties of CME on human colorectal cancer cells have not been extensively studied. Therefore, the study’s objective is to examine the potential of antioxidant and anticancer effects of CME on colon cancer using human cancer cell lines.
MATERIALS AND METHODS
Plant materials
Cordyceps militaris fungus was produced by Ganofarm R&D SDN BHD research laboratory (Puchong, Selangor, Malaysia). The isolate of C. militaris (strain CMRU-1) used in the present study was collected from the Department of Plant Protection, Can Tho University, Vietnam.
Extraction of the sample
Fresh fruiting bodies or mycelia of C. militaris were extracted using the maceration technique. 100 g of the sample was macerated in 1,000 ml of water (stirred at 200 rpm) at 90°C for 1 hour (Azrie et al., 2014; Morales et al., 2019). The crude CME was then filtered. With some modification, the CME was mixed with 10% of maltodextrin and blended until homogeneous (Chankana et al., 2013; Chong and Wong, 2015). The mixture was spray-dried with the inlet and exit air temperatures were 170and 80°C (Chankana et al., 2013). The mixture was sprayed through a 1.5 bar atomizer pressure nozzle during the spray-drying process (Chankana et al., 2013). The spray-dried CME was collected and weighed and the percentage yield was determined. The extracts were stored in the desiccator before further analysis. The yield of the CME was calculated using the following equation:
Total phenolic and total flavonoid content (TFC)
The total phenolic content (TPC) of CME was analyzed using the Folin–Ciocalteu’s method. 1 ml of CME (12.5 mg/ml) was mixed with 50% Folin–Ciocalteu reagent (50 μl) and 2% sodium carbonate (2 ml). The solution was thoroughly mixed before being incubated for 30 minutes at room temperature. Using a UV-Vis spectrophotometer (UV1800, Kyoto, Japan), the solution’s absorbance was measured at 720 nm. Gallic acid was used as a standard and a calibration curve was constructed (6.55–32.79 mg/l). The TPC was measured as a milligram of gallic acid equivalent (GAE) in a gram of dry weight extract. Each experiment was carried out in triplicate, unless otherwise mentioned. The flavonoid–aluminum complex formation was used to assess the TFC of CME. 1 ml of CME (12.5 mg/ml) was combined with 1 ml of methanol and 2% aluminum chloride. After 15 minutes of incubation, the complex was formed and spectrophotometrically analyzed at 430 nm. A standard calibration curve of rutin (6.67–33.33 mg/l) was constructed. TFC was described as a milligram of rutin equivalent (RE) in a gram of dry weight extract.
1, 1-Diphenyl-2-picrylhydrazyl (DPPH) assay
1 ml of sample was thoroughly mixed with 2 ml of DPPH solution (0.1 mM) at concentrations ranging from 100 to 500 μg/ml. After 30 minutes of incubation, the absorption was measured at 520 nm. In this study, rutin was used since it is commonly used as a positive control in all previous antioxidant assays. The potential to scavenge the DPPH was measured using equation (2), where the regulation and the sample absorbance are A control and A sample, respectively, as follows:
Cell culture
Human colorectal cancer cell lines HT-29 were grown in RPMI-1640 (Gibco, Waltham, MA) containing 10% fetal bovine serum (FBS) and 1% of penicillin–streptomycin mixed solution. 0.05% of trypsin-Ethylenediaminetetraacetic acid (EDTA) (GIBCO, Waltham, MA) was used to harvest the confluent cells, which was neutralized with RPMI-1640 supplemented with 10% of FBS (1:1). The cells were routinely cultured in 25 cm2 plastic corning flasks (T-25) and kept at 37°C in a humidified atmosphere with 5% of carbon dioxide supply (CO2) maintained at 37°C as monolayer cultures.
Cytotoxicity assay
To dilute the human cell lines to a concentration of 5 × 103 cells ml−1, serum-free RPMI-1640 (GIBCO, Waltham, MA) was used. A total of 0.1 ml of cell suspension was pipetted into each of the 96-well microtiter plate’s allocated wells. In this study, the blank control group consisted of three wells containing a culture medium. In a 5% CO2 incubator at 37°C, the plate was incubated for 24 hours. The culture medium was pipetted out after incubation, and 0.1 ml of serum-free culture medium containing CME with different concentrations from 0.625 to 10.000 μg/ml was distributed in triplicate into specified wells. The positive control (cisplatin) was used in this study with a concentration of 10 μg/ml (Sigma, Cream Ridge, NJ). For 48 hours, the plate was incubated at 37°C in a 5% CO2 incubator. The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reagent was then applied to each well in a volume of 20 μl. This plate was then incubated in a CO2 incubator at 37°C for another 4 hours before the dye was clear. The supernatant was then was pipetted out, and each well was filled with 0.1 ml of Dimethyl Sulfoxide (DMSO). At 540 nm, the microplate Enzyme-linked immunosorbent Assay (ELISA) reader (Corona Microplate Reader SH1000, Hitachi) was used to measure the absorbance. Equation (3) was used to calculate the concentration inhibition (CI, %) as follows:
CI (%) = 1 – {(As – Ab)/(Ac – Ab)}100 (3)
where the absorbance of blank (Ab), negative control (Ac), and sample (As) were denoted in the equation. Determination of the half-maximal inhibitory concentration (IC50) of each extract was made by interpolating the linear regression of percentage of mortality versus the concentration of extract. The experiment was carried out in triplicate.
Acridine orange (AO) and propidium iodide (PI) double staining
The cell lines at the concentration of 106 cells/ml were treated with CME (at the concentration of the IC50. On the other hand, cells with no application of CME were used as the negative control. Both of the treated and untreated cells were incubated in a 25 cm2 tissue culture flask for 48 hours at 37°C. A 20 μl of AO/PI mixture in PBS at 1:1 (v/v) ratio was used to stain the cells and was visualized using a Leica fluorescence microscope DM 2500 (Leica Microsystem, Wetzlar, Germany) at 100× magnification. Alpha Imager (AlphaInnotech, San Leandro, CA) was used to capture the images of the cells.
RESULT AND DISCUSSION
Antioxidant activity on the DPPH, TPC, and TFC
The percentage yield of spray-dried CME obtained was 10.2 w/w%. Based on the C analysis of variance analysis in Table 1, all the antioxidant activities which were DPPH, TPC, and TFC of CME were significantly (p < 0.05) higher than fresh CM. The higher percentage of inhibition of the antioxidant activity presence and lowest half-maximal IC50 in CME is due to the high content of TPC (160 ± 0.74 mg GAE/100 g) and TFC (6.6 ± 1.13 mg RE/100 g) present in the extract. While CM samples only showed a moderate inhibition on the DPPH activity which was 54.3% (IC50 = 2.95 mg/ml) compared to CME 83.8% (IC50 = 0.60 mg/ml). The CME demonstrated strong antioxidant efficacy by reducing the initial violet color of the DPPH solution to subtle violet and consequently brighter yellow. The DPPH (a stable free radical) is converted to 1,1-diphenyl-2-picrylhydrazyl due to the reaction with antioxidants (Iqbal et al., 2017). The level of discoloration suggests the antioxidant’s radical-scavenging potential. In this step, the DPPH reacts with compounds that convert it to a solid diamagnetic molecule by contributing hydrogen (H) atoms. The antioxidant activity of CME was comparable to Agaricus blazei (97.1% at 2.5 mg/ml), Ramaria botrytis polysaccharides (82.67% at 1.4 mg/ml), and Coprinus comatus (84.5% at 5 mg/ml) (Huang et al., 1999; Li et al., 2017; Tsai et al., 2007).
Cytotoxic effect of CME on HT-29 cells
The MTT cytotoxicity results showed that CME has the highest inhibitory activities followed by synthetic anticancer drug cisplatin in a dose-dependent manner (Fig. 1). On the contrary, cisplatin expressed low inhibitory activities against HT-29 cells. The IC50 values for CME and cisplatin were to be 1.53 and 3.11 mg/ml, respectively. The obvious difference in the mean percentage inhibition of the five concentrations (p < 0.0001) of CME and cisplatin using Tukey’s Honest significant difference (HSD) post-hoc analysis stipulated that the 10 mg/ml of extract had significantly decreased the value of the absorbance as the extract concentration increased. However, the inhibition percentage increases with an increase in CME. This result showed that the compound has a high cytotoxic effect on HT-29 cells even at the lowest concentration of the extract, which is 0.625 and 1.25 mg/ml with 64.66% and 52.14% cell inhibition as compared to cisplatin with 52.52% and 49.14% cell inhibition, considering that the CME is a crude extract.
 | Table 1. The antioxidant activity of CME and fresh CM. [Click here to view] |
The chemopreventive activity of cordycepin, a major compound responsible for anticancer properties in CME against human colon, liver, bladder, and renal cancer, lung, and breast cancer cell lines, has been reported (Choi et al., 2011; Lee et al., 2009; Shao et al., 2016; Tao et al., 2016; Yamamoto et al., 2015; Yoon et al., 2018). Nevertheless, the study has been limited to its effect on mitochondrial dehydrogenase activity. It was confirmed that the crude CME exhibits powerful concentration-dependent growth inhibitory activity against HT-29 cells. Furthermore, the results showed that CME was more cytotoxic against colorectal cancer (IC50 of 1.53 mg/ml) than cisplatin (IC50 = 3.11 mg/ml). Furthermore, changes in the HT-29 cell shape to round in shape (from polygonal shape), reduction of cell adherence (due to cell death), increment of cell debris, and decrease in cell density were observed, indicating the cytotoxicity effect of CME (Fig. 2).
Disruption of cell membrane integrity results in the increment of red fluorescence and the reduction of green fluorescence image in the AO/PI assay. The fluorescent microscopy study was executed to investigate the mode of HT-29 cell death by CME. The HT-29 morphological observation demonstrates features of chromatin condensation and nuclear margination which were the main characteristics of apoptosis together with the loss of cell membrane integrity after 48 hours of incubation. Chromatin condensation and nuclear margination due to apoptotic trigger were observed in both early (indicated by the chromatin condensation and nuclear fragmentation) and late apoptosis (formation of apoptotic bodies and membrane loss) features as shown in Figure 3.
It was observed that the HT-29 cells treated with CME exhibited early apoptotic behavior with the formation of condensed chromatin and marginated nuclear indicated with a bright-green color stain after 48 hours of treatment together with membrane blebbing. In addition, late stages of apoptosis also appeared after the treatment as green-orange fluorescence stain was observed. Treatment with CME also denatures the deoxyribonucleic acid of the cell as observed in red in the morphological image analysis due to the binding with AO.
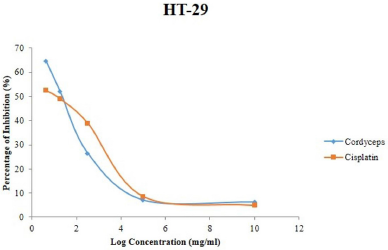 | Figure 1. Viability (%) of HT-29 cells 48 hours after treatment with different concentrations of CME. [Click here to view] |
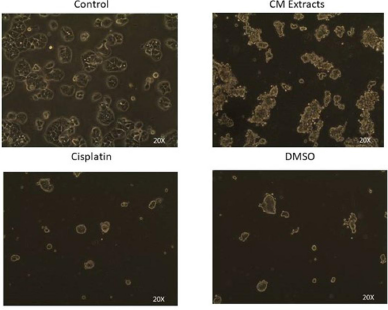 | Figure 2. Morphological changes of HT-29 cancer cell. Cells are imaged by inverted phase-contrast microscope. [Click here to view] |
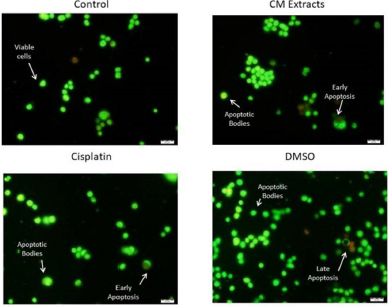 | Figure 3. Morphological changes of HT-29 cancer cell detected with AO/PI staining method and imaged by fluorescence microscope. [Click here to view] |
CONCLUSION
Overall, the CM treatment on colorectal cancer HT-29 cell lines possesses a strong cytotoxic effect in which it has the highest percentage of cytotoxicity with the lowest IC50 value. The high antioxidant content in CM extract shows the parallel agreement with the results of cytotoxic and apoptotic activities of CM extract against HT-29 cells. In conclusion, the results of the present study indicate that CM extract reduces the malignancy of colorectal cancer cells and this anticancer effect of CM extract may present a novel method of treating colorectal cancer and provide evidence on the pharmaceutical potential of CM crude extract as a chemotherapeutic agent against colorectal cancer.
ACKNOWLEDGMENTS
This work is supported by the Tier 1 UTHM Grant (No. 186). The authors would like to express their deepest gratitude to the Faculty of Medicine, Universiti Sultan Zainal Abidin, Universiti Tun Hussein Onn, and Ganofarm R&D SDN BHD, for its facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Azrie AM, Chuah AL, Pin KY, Tan HP. Effect of solvents on the extraction of Kacip Fatimah (Labisia pumila) leaves. J Chem Pharm Res, 2014; 6(9):172–6.
Chankana N, Monton C, Saingam W, Kittiwisut S, Suksaeree J, Sakunpak A, Tengwattanachoti Y. Effect of spray drying carriers on physical properties of spray dried anti-fee-mareng-suang extract powder. Thai J Pharm Sci, 2013; 38:247–50.
Choi S, Lim M, Mo K, Hwa B, Song WO, Woong T. Cordycepin-induced apoptosis and autophagy in breast cancer cells are independent of the estrogen receptor. Toxicol Appl Pharmacol, 2011; 257(2):165–73. CrossRef
Chong SY, Wong CW. Production of spraydried Sapodilla (Manilkara zapota) powder from enzyme-aided liquefied puree. J Food Process Preserv, 2015; 39:2604–11. CrossRef
Huang SJ, Huang LC, Chen CC, Mau JL. Antioxidant properties of Agaricus blazei. In: Proceedings of the third international conference on mushroom biology and mushroom products (Eds, Broderick A & Nair T), Sydney, Australia, 1999, p 266.
Iqbal S, Sivaraj C, Gunasekaran K. Antioxidant and anticancer activities of methanol extracts of seeds of Datura stramonium. Free Radic Aantioxidants, 2017; 7:184. CrossRef
Joshi M, Sagar A. In vitro free radical scavenging activity of a wild edible mushroom, Sparassis crispa (Wulf.)Fr., from north western Himalayas, India. J Mycol, 2014; 2014:1. CrossRef
Kim HW, Kim YH, Cai XF, Nam KS, Lee SJ, An HS. In vitro antitumor activity of ergosterol peroxide isolated form Cordyceps militaris on cancer cell lines from Korean patients. Korean J Mycol, 2011; 29:61−6.
Lee H, Kim YJ, Kim HW, Lee DH, Sung MK, Park T. Induction of apoptosis by Cordyceps militaris through activation of caspase-3 in leukemiaHL-60 cells. Biol Pharm Bull, 2006; 29:670–4. CrossRef
Lee S, Kim S, Choi W, Kim W, Moon S. Cordycepin causes p21WAF1-mediated. G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch Biochem Biophys, 2009; 490(2):103–9. CrossRef
Li H. Extraction, purification, characterization and antioxidant activities of polysaccharides from Ramaria botrytis (Pers.) Ricken. Chem Cent J, 2017; 11:24. CrossRef
Lim HW, Kwon YM, Cho SM, Kim JH, Yoon GH. Antitumor activity of Cordyceps militaris on human cancer cell line. Korean J Pharmacogn, 2009; 35:364–7.
Lim HW, Kwon YM, Cho SM, Kim JH, Yoon GH, Lee SJ. Antitumor activity of Cordyceps militaris on human cancer cell line. Korean J Pharmacogn, 2004; 35:364−7.
Morales D, Smiderle FR, Piris AJ, Soler-Rivas C, Prodanov M. Production of a β-D-glucan-rich extract from Shiitake mushrooms (Lentinula edodes) by an extraction/microfiltration/reverse osmosis (nanofiltration) process. Innov Food Sci Emerg Technol, 2019; 51:80–90. CrossRef
Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol, 2005; 57(12):1509–19. CrossRef
Palacios I, Lozano M, D’Arrigo M, Rostagno MA, Martinez JA, Garcia Lafuente A, Guillamon E, Villares A. Antioxidant properties of phenolic compounds occurring in edible mushroom. Food Chem, 2011; 128:674. CrossRef
Park C, Hong SH, Lee JY. Growth inhibition of U937 leukemia cells by aqueous extract of Cordyceps militaris through induction of apoptosis. Oncol Rep, 2005; 13:1211–6. CrossRef
Park SE, Yoo HS, Jin CY, Hong SH, Lee YW, Kim BM. Induction of apoptosis and inhibition of telomerase activity in human lung carcinoma cells by the water extract of Cordyceps militaris. Food Chem Toxicol, 2009; 47:1667–75. CrossRef
Shao LEWEN, Huang LIHUA, Yan S, Jin JDI, Ren SYAN. Cordycep in induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signaling pathways. Oncol Lett, 2016; 12(2):995–1000. CrossRef
Shrestha B, Sung JM. Notes on Cordyceps species collected from the central region of Nepal. Mycobiology, 2005; 33(4):235–9. CrossRef
Tao X, Ning Y, Zhao X, Pan T. The effects of cordycepin on the cell proliferation, migration and apoptosis in human lung cancer cell lines A549 and NCI-H460. J Pharm Pharmacol, 2016; 68:901–11. CrossRef
Tsai SY, Tsai HL, Mau JL. Antioxidant properties of Agaricus blazei, Agrocybe cylindracea and Boletus edulis. LWT-Food Sci Technol. 2007; 40:1392. CrossRef
Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci, 2013; 93:863–9. CrossRef
Wong KL, So EC, Chen CC, Wu RSC, Huang BM. Regulation of steroidogenesis by Cordyceps sinensis mycelium extracted fractions with (hCG) treatment in mouse Leydig cells. Arch Androl, 2007; 53(2):75–7. CrossRef
Xiao JH, Qi Y, Xiong Q. Nucleosides, a valuable chemical marker for quality control in traditional Chinese medicine Cordyceps. Recent Pat Biotechnol. 2013; 7(2):153–66. CrossRef
Yamamoto K, Shichiri H, Uda A, Yamashita K, Nishioka T, Kume M, Hirai M. Apoptotic effects of the extracts of Cordyceps militaris via Erk phosphorylation in a renal cell carcinoma cell line. Phytother Res, 2015; 713:707–13. CrossRef
Yoon SY, Park SJ, Park YJ. The anticancer properties of cordycepin and their underlying mechanisms. Int J Mol Sci, 2018; 19(10):3027. CrossRef
Yu HM, Wang BS, Huang SC, Duh PD. Comparison of protective effects between cultured Cordyceps militaris and natural Cordyceps sinensis against oxidative damage. J Agric Food Chem, 2006; 54(8):3132–8. CrossRef
Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol, 2013; 65:474–93. CrossRef
Zhou X, Gong Z, Su Y, Lin J, Tang K. Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol, 2009; 61:279–91 CrossRef