INTRODUCTION
Cognition, an exclusive function of the brain, is the sum total of mental activities involved in thinking, reasoning, learning, and memory regulation. Impairment of this function is well known as cognitive impairment (CI) disease (Deture and Dickson, 2019; Johansson et al., 2015). This condition is characterized by impairment in attention and focus, calculation ability, decision-making, thinking, and memory. CI is a complex and progressive disease caused by many factors. Many studies describe aging as a major risk factor in CI. The prevalence of CI was reported to increase linearly with increasing age (19.2% at 65–74 years old, 27.6% at 75–84 years old, and 38% at 85 years or older). There are more than 16 million people in the USA with CI, and almost 5.1 million have Alzheimer’s disease (AD), the most common type of CI. This number is predicted to be tripled or around 152 million people in 2050, especially in low- and middle-income countries (Li et al., 2020; Richardson et al., 2019).
The incidence of CI is also associated with several serious and mental diseases, such as AD (8.2%), stroke or cerebrovascular diseases (5.7%), and alcohol abuse (1.5%). The mortality rate of CI is 8%, and it tends to increase annually. Although stroke and AD are the two diseases with the fastest annual progression (20% and 17%, resp.), the annual progression of CI to dementia is still high (11.7%). In addition, CI has many negative impacts on health status, independence, and socioeconomic aspects of human beings. CI is considered as a high-cost illness from a socioeconomic perspective. Previous clinical studies in the USA reported that AD and dementia are the third most expensive diseases, costing approximately nine times more than other diseases. Globally, the costs of AD and dementia reached as much as US$ 2 trillion in 2030 (Rizzi et al., 2014). Thus, a therapeutic approach preventing or curing CI is crucial to maintain and even increase the health status of the global community. To date, the clinical effectiveness of conventional drugs for the treatment of CI (e.g., tacrine, donepezil, rivastigmine, and galantamine) is still limited. These drugs failed to provide consistent efficacy across all cases of CI. Moreover, undesirable side effects (e.g., nausea, vomiting, hepatotoxicity, and diarrhea) often accompanied the main therapeutic effect in long-term medication (Mehta et al., 2012; Sharma et al., 2019; Tiwari et al., 2019). This emphasizes the need for discovering alternative therapeutic agents for CI with high efficacy and minimal side effects.
One of the potential sources of agents to prevent or cure neurodegenerative diseases is medicinal plants. Plants provide bioactive natural compounds with wide structural diversity that might match the therapeutic targets of CI and other neurological disorders (Lautie et al., 2020). Many studies have been conducted to explore the potential of medicinal plants for the treatment of CI using different targets and mechanisms of action. Additionally, some studies demonstrated the chemical constituents responsible for the activity of such plants and their therapeutic targets. The mechanisms underlying the pharmacological effects to explain how the phytochemical constituents exert their effects were also reported.
In this review article, we summarize the cognitive-enhancing effects of plant natural compounds from preclinical studies. The therapeutic targets or modes of action in the context of CI are also discussed in this article. To provide a scientific basis for CI therapy and a better understanding of the therapeutic targets, the pathophysiology of CI was also briefly introduced.
Pathophysiology of CI
Previous studies suggested that several abnormal conditions of the central nervous (CNS) are strongly correlated with the pathology of CI in humans (Adams et al., 2017; Mufson et al., 2012). We have summarized these with an emphasis on the four conditions explained below as a suggested model of the underlying mechanism of CI pathophysiology (Fig. 1).
Aging and CI
Aging is a natural physiological process closely related to decreased human quality of life and increased complex disease risk factors, including neurodegenerative disorders. The majority of elderly people demonstrate a decrease in the endogenous immune system and antioxidant systems. These conditions lead to inflammatory reactions, aging, and oxidative stress, which cause impairment of brain neurons (Fard and Con, 2019).
Amyloid beta (Aβ) and tau proteins in CI
Aβ plaque is a toxic protein and represents a hallmark of AD. This insoluble protein is a product of amyloid precursor protein (APP) degradation by the enzyme secretase. The three types of Aβ protein are Aβ monomer, dimer, and oligomer. Among them, the oligomer Aβ is the most toxic to the brain. The oligomer AB can reside in several regions of the brain, such as the basal ganglia, thalamus, hypothalamus, medulla oblongata, and cerebellum. Additionally, neurofibrillary tangles (NFT), a misfolded form of tau protein, are also found and can lead to CI and other brain diseases. In the normal condition, tau protein is a substantial protein that plays a role in the stabilization of the microtubules of neurons. This protein is part of the neuron and responsible for maintaining nutrients and transporting substances required by the brain. The accumulation of Aβ plaque and NFT in the temporal and frontal cortex regions leads to synaptic dysfunction and further provokes CI in the patients with AD via oxidative stress and neuroinflammatory mechanisms (Deture and Dickson, 2019; Tönnies et al., 2017; Kent et al., 2020; Tiwari et al., 2019).
 | Figure 1. Pathophysiology of CI. Oxidative stress and neuroinflammation are the two main events leading to CI. The other factors associated with the pathophysiology of CI are the presence of Aβ and tau protein, cerebral hyperperfusion, aging, and neurotransmitter disturbances. These factors lead to neural loss and memory/learning deficits leading to CI. [Click here to view] |
Cerebral hypoperfusion and CI
CI is commonly found in poststroke and/or traumatic brain injury conditions. Hemodynamic abnormalities, particularly in cerebral hypoperfusion, are associated with neurodegeneration in these conditions. Hypoperfusion of the cerebri causes imbalances of endogenous reactive oxygen species/nitrite oxygen species (ROS/NOS)-antioxidant systems and leads to oxidative brain injury. This condition activates microglia to release a number of proinflammatory cytokines and induces a severe neuronal loss in the brain (Liu and Zhang, 2012).
Neurotransmitter disturbances and CI
Disturbances in acetylcholine (ACh), serotonin (5HT), dopamine (DA), and glutamate (Glu) neurotransmitters contribute to memory and learning deficiencies and cause CI. Decreasing ACh, 5HT, and DA levels in the brain are closely correlated with AD and Parkinson’s disease. The low level of these neurotransmitters in the brain is caused by aberrations in their production located in the presynapse and/or by degradation in the synaptic junction. This condition interferes with the transmission of nerve impulses and impairs cognitive functional signaling pathways. In contrast to the neurotransmitters mentioned previously, high levels of Glu induce calcium neuroexcitotoxicity through persistent activation of N-methyl-d-aspartate acid (NMDA) and α-amino-3-hydroxy-5-methylisoxazole propionic acid receptors and cause neuronal damage (Yunqi et al., 2013).
Oxidative stress and neuroinflammation as a major pathogenetic mechanism of CI
Imbalances of the endogenous antioxidant system are reported as one of the major causes of progressive neurodegenerative diseases. In this case, overproduction of ROS/NOS causes oxidative stress that triggers lipid peroxidation and induces neuronal damage in the brain (Tönnies and Trushina, 2017). Neuroinflammation is the body’s response to the accumulation of Aβ plaque and is recognized as a common feature of AD. Neuroinflammation is considered as a key factor in the pathogenesis and progression of AD. Neuroinflammation is initiated by the activation of microglia, which induces the release of proinflammatory cytokines, such as IL-6 and tumor necrosis factor alpha (TNFα) (Kinney et al., 2018). An understanding of the pathophysiology of neuroinflammation is crucial for identifying potential therapeutic targets in the effort to discover and develop cognitive-enhancing drugs.
Potential therapeutic targets in CI
There are four potential therapeutic targets for the prevention and treatment of CI. These therapeutic targets are shown in Figure 2.
Neurotransmitter modulators
The common target of cognitive function-enhancing drugs is the inhibition of cholinesterase (ChE) and monoamine oxidase enzymatic activity, as well as the inhibition of the enzymes responsible for ACh and monoamine neurotransmitter degradation. These so-called “neurotransmitter modulator” drugs effectively increase intracellular levels of ACh, 5HT, and DA. In memory and learning ability, neurotransmitter modulators are required to initiate neurotransmitter–receptor binding postsynapse, which stimulates various cellular and molecular signal transductions to improve cognitive function, regulation, and maintenance (Ferreira-Vieira et al., 2016; Hampel et al., 2020; Stanciu et al., 2019). Interestingly, drugs that antagonize ChE and NMDA are ineffective at stopping the progression of CI. However, the clinical use of these drugs is proven to be effective at improving cognitive performance and other symptoms of CI only for a short time period (Moss, 2020; Yaari and Ann, 2015).
 | Figure 2. Potential therapeutic targets in CI. Antioxidant, anti-inflammatory, antitau, antiamyloid, and neurotransmitter modulator represent the promising therapeutic target of plant natural products for combating CI. [Click here to view] |
Antiamyloidogenic
Antiamyloidogenic is a term to describe a group of drugs or substances that inhibit Aβ plaque formation, aggregation, and fibrillation, as well as promoting Aβ plaque degradation and clearance. Antiamyloidogenic agents act by downregulating β and γ-secretase and upregulating α-secretase enzyme activity. The decrease in Aβ plaque accumulation potentially reduces the risk of neuroinflammation, which represents the main factor causing AD. Antiamyloidogenic drugs are a relatively novel and promising approach for the treatment of AD and other forms of dementia. The development of these drugs is challenging, especially in the clinical trial stage (Yaari and Ann, 2015). Although the therapeutic approach targeting Aβ production and deposition is a promising hypothesis, none of the clinical trials so far succeeded in developing effective and safe therapeutic agents. The clinical outcome of this agent is determined by various factors that affect its efficacy and safety. These factors include the intrinsic factors such as polarity and molecular size that dictate the ability to cross the blood–brain barrier and the extrinsic factors such as the genetics of patients, severity of illness, and neuropathology. The clinical trials of the agents targeting Aβ such as lanabecestat, semagacestat, verubecestat, atabecestat, aducanumab, bapineuzumab, solanezumab, crenezumab, and gantenerumab have failed due to the lack of efficacy and the emergence of toxic effects (Abushakra et al., 2017; Tolar et al., 2020; Oxford et al., 2020).
Antioxidant agents
The consumption of dietary supplements comprising antioxidative agents is an appropriate approach to overcoming endogenous antioxidant system imbalances and/or insufficiencies. Antioxidants are required to improve the body’s defense system to prevent neuronal loss due to lipid peroxidation in the brain. They prevent the loss of neuron and synapse degeneration in the median temporal lobe, hippocampus, and cortex. Thus, decreases in neurotransmitter levels in the brain can be avoided. Intake of antioxidant compounds can protect the brain from the oxidative damage associated with AD. Antioxidants directly or indirectly inhibit ROS/NOS formation and modulate the activity and expression of endogenous antioxidants. Many studies have demonstrated that the consumption of polyphenol compounds with antioxidant activity is associated with a lower risk and slower progression of AD (Colizzi, 2019).
Anti-inflammatory drugs
The efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) for improving CI in AD is still a matter of debate. Preclinical evidence revealed that the use of NSAIDs is a promising therapeutic approach for the prevention and treatment of AD. As mentioned earlier, chronic neuroinflammation is a well-known attribute of AD and is involved in its pathogenesis. The underlying mechanism of this drug is related to the inhibition of neuroinflammatory progression upon the occurrence of Aβ plaques and NFT in the brain. However, the administration of anti-inflammatory drugs showed modest efficacy, and this effect is inconsistent in the clinical context. NSAIDs effectively reduce the risk of AD and dementia, but only in the early stage of the diseases (Imbimbo et al., 2010). A recent clinical investigation showed that the use of several NSAIDs, especially diclofenac, was associated with a reduction of the prevalence and progression rate of CI in AD (Imbimbo et al., 2010; Stuve et al., 2020).
Potential natural cognitive enhancers from plants
Medicinal plants have been used traditionally to treat cognitive-related diseases, including cognitive disorders. The long history of drug development from natural products proves that many natural compounds of plant origin have inspired the discovery of new drug entities or “lead compounds” (Achilonu and Dennis, 2015; Rahimi et al., 2010). For example, physostigmine isolated from Physostigma venenosum seeds demonstrated parasympathomimetic activity in the human CNS system. Physostigmine was the first drug candidate for the treatment of AD and parasympathetic-related diseases, such as myasthenia gravis and glaucoma. Unfortunately, this development was hampered by strong scientific evidence indicating that physostigmine has a narrow therapeutic index and a short duration of action and shows undesired side effects, such as abdominal colic, nausea, vomiting, hypersalivation, and hyperhidrosis. Later, the chemical structural modification of physostigmine resulted in the development of the new drug entities epastigmine and fenserine. Another example of a promising natural compound for the treatment of AD is galantamine, an alkaloid isolated from the bulb of Galanthus nivalis. Galantamine potently inhibited AChEI and showed efficacy against AD (Hermann, 2015; Mehta et al., 2012).
Nowadays, drug discovery efforts are drawing major attention by focusing on the identification of bioactive compounds of plant origin, including those for cognitive function-enhancing drugs (Benek et al., 2020). A cognitive function enhancer, also known as a nootropic or “smart drug,” is a synthetic and/or natural substance that is used to improve cognitive functions. This drug is widely used for the treatment of neurodegenerative diseases, and it effectively enhances cognition aspects in patients with AD and other cognitive function-related disorders affecting memory, learning ability, motivation, attention, and focus. Several natural compounds that have been tested for cognitive function-enhancing activity in an amnesic-animal model are presented in Table 1.
Table 1 shows that scopolamine-induced memory loss in rodents is the most popular bioassay used by researchers for evaluating cognitive function-enhancing effects. Scopolamine is a muscarinic receptor antagonist that acts by blocking the central cholinergic system and the nervous system. Blockade of this system leads to CI (especially in learning and memory ability), which is the hallmark of AD (Balmus and Ciobica, 2017; Blokland et al., 2016; Jivad and Rabiei, 2014; Prashar et al., 2014). Additionally, scopolamine is well known as a potent inducer of ROS/NOS in the upregulation of proinflammatory cytokines in the CNS (Haider et al., 2016). These conditions trigger neuronal damage leading to AD. Based on the bioactive compound variability presented in Table 1, we clustered the active compounds on the basis of their chemical structure. Figure 3 shows that the chemical classes of the active compounds are very diverse, ranging from simple to complex. Interestingly, flavonoids are the most frequently reported compounds for enhancing cognitive function, followed by terpenoids and alkaloids.
A flavonoid is a secondary metabolite compound with a C6-C3-C6 backbone. Flavonoids are widely distributed in plants and have various biological activities. Although most flavonoids show antioxidant activity due to the presence of hydroxyl groups (Brodowska, 2017; Kumar and Abhay, 2013), some flavonoids (e.g., curcumin, ellagic acid, genistein, kolaviron, luteolin, myricetin, oroxylin A, quercetin, resveratrol, trans-cinnamaldehyde, vitexin, and mangiferin) exert cholinomimetic activity and block the cholinergic system. Flavonoids reduce oxidative stress and inflammation and might thus lower the risk of memory impairment. These lines of evidence suggest that flavonoids are promising natural compounds for further development as drugs for AD. Other flavonoids, such as hesperidin, rutin, anthocyanins, naringin, and silibinin, are the most reported flavonoids tested for their therapeutic value in AD using in vivo models (de Andrade Teles et al., 2018).
Multitarget action of natural compounds for CI
Many studies have demonstrated the multitarget actions of herbal medicines and their metabolites, which is important for drug discovery and development efforts (Bizzarri et al., 2020). A multitarget drug is a new perspective on modern drug design, especially for combating complex diseases, including neurodegenerative diseases (AD, Parkinson’s disease, schizophrenia, and depression) and cancers. These diseases have multiple pathophysiological and pathological aspects manifested in their clinical symptoms. Therefore, a single target drug might be inadequate to effectively achieve the therapeutic goal (Ramsay et al., 2018). Consequently, an effective drug might be developed on the basis of multiple targets to cover complex therapeutic targets.
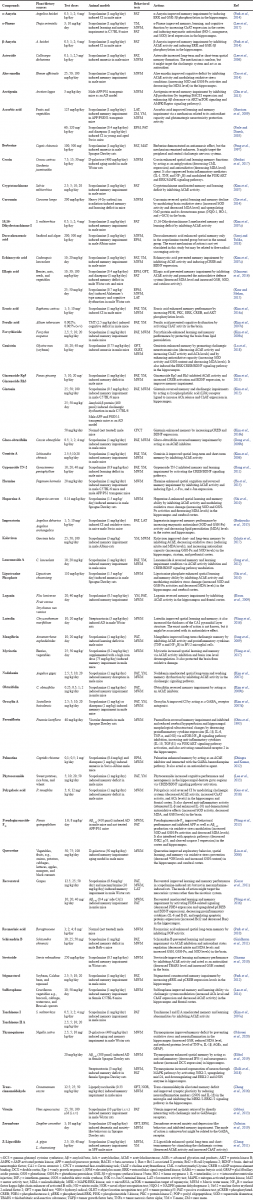 | Table 1. Natural compounds with potential cognitive function-enhancing activity evaluated in amnesic-animal models. [Click here to view] |
As a recent study indicated that CI is a complex disease involving genetic, environmental, and aging factors with complicated pathophysiology (Alber, 2017; Sun et al., 2017; Tiwari et al., 2019), a multitarget approach is needed for the development of CI drugs. In this regard, plant natural compounds represent a potential source. Plants serve compound diversity that is historically proven to inspire drug discovery and traditionally used for medicinal purposes in various diseases, including complex ones (Benek et al., 2020; Chen and Decker, 2013).
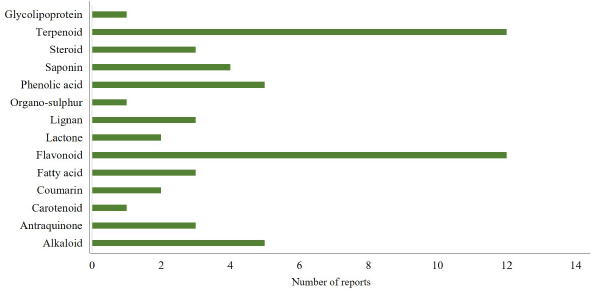 | Figure 3. Chemical classes of compounds acting as memory enhancers in animal studies published from 2003 to 2020. Among the group of compounds, flavonoid and terpenoid are the most frequently reported memory enhancers, whereas only little data are available for carotenoid and organosulfur compounds. [Click here to view] |
 | Figure 4. Comparison of the mechanism of action of plant natural products acting as cognitive enhancers evaluated in animal models. Modulation of cholinergic activity accounted for 34% of the mechanism, whereas oxidative stress modulation and signaling pathway activation accounted for 19% and 14%, respectively. The other mechanism of actions was accounted less than 10%. [Click here to view] |
As previously shown in Table 1, many plant-derived natural compounds exhibited cognitive-enhancing activity in amnesic-animal models. Their potential effects were evaluated by behavioral testing, employing short and/or long-term spatial and working memory performance evaluations. Memory is an important factor in cognition function, and impairment of cognitive function is associated with the early stage of cognitive problems (Robertson, 2002). Based on Table 1, plant natural compounds were grouped on the basis of their mechanisms of action (Fig. 4). Figure 4 shows that the cholinergic nervous system is the major target of the majority of the potential natural compounds, followed by ROS/NOS and some of the signaling pathways in the CNS for their activity.
Fifteen compounds showed multitarget action, by at least three different mechanisms. These compounds were gintonin, thymoquinone, huperzine A, aloe emodin, curcumin, ellagic acid, genistein, kolaviron, resveratrol, stevioside, schisandrin B, sulforaphane, echinocystic acid, lancemaside A, and polygalacic acid, and they have a high potential to be further developed as drugs targeting CI. Polygalacic acid and genistein are the most potential and promising candidates as a new cognitive function-enhancing drug. These compounds can be found in Polygala tenuifolia and Glycine max (soybean). Polygalacic acid (3; 6; 12 mg/kg; p.o) and genistein (10; 20; 40 mg/kg and 10; 20; 40 mg/kg; p.o) were able to inhibit memory impairment in mice induced by scopolamine. Polygalacic acid and genistein showed a synergistic effect in targeting CI. These compounds have several mechanisms (i.e., regulating the cholinergic nervous system, activating the extracellular signal-regulated kinase (ERK)/cAMP response element binding (CREB)/brain- derived neurotrophic factor (BDNF) signaling pathway, and protecting the hippocampus and frontal cortex from oxidative and inflammatory stresses). The activity of polygalacic acid and genistein is of note, as it protects the hippocampus and frontal cortex (the most important regions of CNS for cognition regulation) from injuries and stresses. However, further studies are required to clarify their antiamnesic activity in human using clinical trials (Guo et al., 2016; Lu et al., 2018).
The limitations of natural cognitive enhancers from plants
Plant natural compounds have a potential to be developed as a cognitive-enhancing agent. They provide a huge chemical diversity and might offer an alternative therapeutic approach. On the contrary, plant natural compounds have some limitations that restrict their development as a drug. Several clinical evidences indicated that herbal medicines and their metabolite constituents demonstrated inconsistent clinical outcomes. This due to the unclear pharmacokinetic aspect of the active compound, poor bioavailability, and lack of penetration across the blood–brain barrier. Consequently, these compounds failed to achieve the minimum therapeutic concentration in CNS, leading to the lack of efficacy. In addition, variability in the quality of plant raw material, harvesting process, extraction method, and production process also affect the quality of the final product (Kunle et al., 2012; Ratheesh et al., 2017).
CONCLUSION
Many studies showed that plant natural compounds have a positive influence on cognitive performance in animal experimental models. These compounds were able to improve cognitive functions and to enhance short/long-term spatial and working memories. Based on the current literature, we identified 15 plant natural compounds that showed multitarget action for combating CI. Polygalacic acid and genistein are among the most promising of these compounds, as they are able to interact with multiple molecular targets related to CI and are considered as promising lead compounds for drug development and dietary supplementation in the treatment of CI.
ACKNOWLEDGMENTS
The authors thank to the Ministry of Research, Technology and Higher Education (KEMENRISTEKDIKTI), through the scholarship of Program Magister menuju Doktor untuk Sarjana Unggul (PMDSU) with contract number 5839/UN1.DITLIT/DIT-LIT/LT/2018 for the financial support during the research and preparation of this manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve the use of animals or human subjects.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbasi E, Nassiri-Asl M, Sheikhi M, Shafiee M. Effects of vitexin on scopolamine-induced memory impairment in rats. Chin J Physiol, 2013; 56(3):184–9.
Abushakra S, Porsteinsson A, Scheltens P, Sadowsky C, Vellas B, Cummings J, Gauthier S, Hey JA, Power A, Wang P, Shen L, Tolar M. Clinical effects of tramiprosate in APOE4/4 homozygous patients with mild Alzheimer's disease suggest disease modification potential. J Prev Alzheimers Dis, 2017; 4:149–56.
Achilonu MC, Dennis OU. Bioactive phytochemicals: bioactivity, sources, preparations and/or modifications via silver tetrafluoroborate mediation. J Chem, 2015; 2015:1–22. CrossRef
Adams PW, Adejare A, Adeniji AO, Alamri M, Ates-Alagoz Z, Barrett JE, Caito SW, Camacho JB, Carlson E, Colestock T, Corum E, Finn LA, Glenn M, Hu DD, Hu C, Khalaj S, Mace L, McGonigle P, Mody VV, Moelter ST, Mondragón-Rodríguez S, Newell-Caito JL, Peña-Ortega F, Perry G, Sheerin M, Taghibiglou C, Valasani KR, Vangavaragu JR, Wallach J, Yacoubian TA, Yan SSD. Drug discovery approaches for the treatment of neurodegenerative disorders: Alzheimer's disease. Elsevier, San Diego, CA, 2017.
Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk : a review and synthesis. Alzheimer's Dement Diagn Assess Dis Monit, 2017; 7:69–87. CrossRef
Balmus IM, Ciobica A. Main plant extracts' active properties effective on scopolamine-induced memory loss. Am J Alzheimers Dis Other Demen, 2017; 32(7):418–28. CrossRef
Benek O, Jan K, Ondrej S. A perspective on multi-target drugs for Alzheimer’s disease. Trends Pharmacol Sci, 2020; 41(7):434–45. CrossRef
Bizzarri M, Giuliani A, Monti N, Verna R, Pensotti A, Cucina A. Rediscovery of natural compounds acting via multitarget recognition and noncanonical pharmacodynamical actions. Drug Discov Today, 2020; 25(5):920–7. CrossRef
Blokland A, Anke S, Jos P, Wim JR. Why an M1 antagonist could be a more selective model for memory impairment than scopolamine? Front Neurol, 2016; 7(167):10–3. CrossRef
Brodowska KM. Natural flavonoids: classification, potential role, and application of flavonoid analogues. Eur J Biol Res, 2017; 7(2):108–23.
Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology, 2015; 232(5):931–42. CrossRef
Chen X, Decker M. Multi-target compounds acting in the central nervous system designed from natural products. Curr Med Chem, 2013; 20(13):1673–85. CrossRef
Cheng LL, Chen XN, Wang Y, Yu L, Kuang X, Wang LL, Yang W, Du JR. Z-ligustilide isolated from radix angelicae sinensis ameliorates the memory impairment induced by scopolamine in mice. Fitoterapia, 2011; 82(7):1128–32. CrossRef
Colizzi C. The protective effects of polyphenols on Alzheimer’s disease: a systematic review. Alzheimer's Dement Transl Res Clin Interv, 2019; 5:184–96. CrossRef
Dalli T, Merve B, Sule TU, Fahri A, Birsen E. Thymoquinone activates MAPK pathway in hippocampus of streptozotocin-treated rat model. Biomed Pharmacother, 2018; 99:391–401. CrossRef
De Andrade Teles RB, Diniz TC, Pinto TCC, de Oliveira Júnior RG, Silva MGE, de Lavor ÉM, Fernandes AWC, de Oliveira AP, de Almeida Ribeiro FPR, da Silva AAM, Cavalcante TCF, Júnior LJQ, da Silva Almeida JRG. Review article flavonoids as therapeutic agents in Alzheimer’s and parkinson’s diseases: a systematic review of preclinical evidences. Oxid Med Cell Longev, 2018; 2018:1–21. CrossRef
Deture MA, Dennis WD. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener, 2019; 14(1):1–18. CrossRef
Dhingra D, Varun K. Memory-enhancing activity of palmatine in mice using elevated plus maze and morris water maze. Adv Pharmacol Sci, 2012; 2012:1–7. CrossRef
Elibol B, Sule TU, Merve B, Cigdem S. Thymoquinone (TQ) demonstrates its neuroprotective effect via an anti-inflammatory action on the Aβ(1-42)-infused rat model of Alzheimer's disease. Psychiatry Clin Psychopharmacol, 2019; 29(4):379–86. CrossRef
Fard MT, Con S. A review and hypothesized model of the mechanisms that underpin the relationship between inflammation and cognition in the elderly. Front Aging Neurosci, 2019; 11(3):1–22. CrossRef
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol, 2016; 14(1):101–15. CrossRef
Gacar N, Mutlu O, Utkan T, Celikyurt IK, Gocmez SS, Ulak G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and morris water maze tests in rats. Pharmacol Biochem Behav, 2011; 99(3):316–23. CrossRef
Giridharan VV, Thandavarayan RA, Sato S, Ko KM, Konishi T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from schisandra chinensis in mice. Free Radic Res, 2011; 45(8):950–8. CrossRef
Guo C, Shen J, Meng Z, Yang X, Li F. Neuroprotective effects of polygalacic acid on scopolamine-induced memory deficits in mice. Phytomedicine, 2016; 23(2):149–55. CrossRef
Haider S, Tabassum S, Perveen T. Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: a comparative study. Brain Res Bull, 2016; 127:234–47. CrossRef
Hampel H, Mesulam M, Claudio CA, Martin RF, Ezio G, George TG, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain, 2020; 141(7):1917–33. CrossRef
Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res, 2009; 205(2):550–8. CrossRef
He D, Wu H, Wei Y, Liu W, Huang F, Shi H, Zhang B, Wu X, Wang C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur J Pharmacol, 2015; 768:96–107. CrossRef
Heidari S, Mehri S, Hosseinzadeh H. Memory enhancement and protective effects of crocin against d-galactose aging model in the hippocampus of Wistar rats. Iran J Basic Med Sci, 2017; 20(11):1250–9.
Hermann AMM. The case of galantamine: repurposing and late blooming of a cholinergic drug. Future Sci OA, 2015; 1(4):FSO73. CrossRef
Hong SW, Yang JH, Joh EH, Kim HJ, Kim DH. Gypenoside TN-2 ameliorates scopolamine-induced learning deficit in mice. J Ethnopharmacol, 2011; 134(3):1010–3.
Imbimbo, BP, Solfrizzi V, Panza F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Front Aging Neurosci, 2010; 2(19):1–14.
Ishola IO, Adamson FM, Adeyemi OO. Ameliorative effect of kolaviron, a biflavonoid complex from garcinia kola seeds against scopolamine-induced memory impairment in rats: role of antioxidant defense system. Metab Brain Dis, 2017; 32(1):235–45.
Jafarian S, King HL, Zurina H, Lua PL, Mohd RS, Enoch KP. Effect of zerumbone on scopolamine-induced memory impairment and anxiety-like behaviours in rats. Alzheimer's Dement Transl Res Clin Interv, 2019; 5:637–43.
Jivad N, Rabiei Z. Study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac J Trop Biomed, 2014; 4(10):780–9.
Johansson MM, Jan M, Ewa W. Cognitive impairment and its consequences in everyday life: experiences of people with mild cognitive impairment or mild. Int Psychogeriatrics, 2015; 2:1–10.
Jung IH, Se EJ, Eun HJ, Jayong C, Myung JH, Dong HK. Lancemaside a isolated from codonopsis lanceolata and its metabolite echinocystic acid ameliorate scopolamine-induced memory and learning deficits in mice. Phytomedicine, 2012; 20(1):84–8.
Jung K, Lee B, Han SJ, Ryu JH, Kim DH. Mangiferin ameliorates scopolamine-induced learning deficits in mice. Biol Pharm Bull, 2009; 32(2):242–6.
Kaur R, Mehan S. Ameliorative treatment with ellagic acid in scopolamine induced Alzheimer’s type memory and cognitive dysfunctions in rats. Austin J Clin Neurol, 2015; 2(6):1–11.
Kent SA, Spires-Jones TL, Durrant CS. The physiological roles of tau and Aβ: implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol, 2020; 140(4):417–47.
Kim DH, Hung TM, Bae KH, Jung JW, Lee S, Yoon BH, Cheong JH, Ko KH, Ryu JH. Gomisin a improves scopolamine-induced memory impairment in mice. Eur J Pharmacol, 2006; 542(1–3):129–35.
Kim DH, Hyun SK, Yoon BH, Seo JH, Lee KT, Cheong JH, Jung SY, Jin C, Choi JS, Ryu JH. Gluco-obtusifolin and its aglycon, obtusifolin, attenuate scopolamine-induced memory impairment. J Pharmacol Sci, 2009b; 111(2):110–6.
Kim DH, Jeon SJ, Jung JW, Lee S, Yoon BH, Shin BY, Son KH, Cheong JH, Kim YS, Kang SS, Ko KH, Ryu JH. Tanshinone congeners improve memory impairments induced by scopolamine on passive avoidance tasks in mice. Eur J Pharmacol, 2007e; 574(2–3):140–7.
Kim DH, Jeon SJ, Son KH, Jung JW, Lee S, Yoon BH, Lee JJ, Cho YW, Cheong JH, Ko KH, Ryu JH. The ameliorating effect of oroxylin a on scopolamine-induced memory impairment in mice. Neurobiol Learn Mem, 2007d; 87(4):536–46.
Kim DH, Kim DY, Kim YC, Jung JW, Lee S, Yoon BH, Cheong JH, Kim YS, Kang SS, Ko KH, Ryu JH. Nodakenin, a coumarin compound, ameliorates scopolamine-induced memory disruption in mice. Life Sci, 2007c; 80(21):1944–50.
Kim E, Ko HJ, Jeon SJ, Lee S, Lee HE, Kim HN, Woo ER, Ryu JH. The memory-enhancing effect of erucic acid on scopolamine-induced cognitive impairment in mice. Pharmacol Biochem Behav, 2016a; 142:85–90.
Kim EJ, Jung IH, Van Le TK, Jeong JJ, Kim NJ, Kim DH. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol, 2013; 146(1):294–9.
Kim HJ, Eun-Joo S, Byung-Hwan L, Sun-Hye C, Seok-Won J, Ik-Hyun C, Hwang SH, Kim JY, Han JS, Chung C, Jang CG, Rhim H, Kim HC, Nah SY. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid β protein, and mouse model of alzheimer's disease. Mol Cells, 2015; 38(9):796–805.
Kim MJ, Choi SJ, Lim ST, Kim HK, Heo HJ, Kim EK, Jun WJ, Cho HY, Kim YJ, Shin DH. Ferulic acid supplementation prevents trimethyltin-induced cognitive deficits in mice. Biosci Biotechnol Biochem, 2007b; 71(4):1063–8.
Kim S, Kim DH, Choi JJ, Lee JG, Lee CH, Park SJ, Jung WY, Park DH, Ko KH, Lee SH, JH, Ryu SH. Forsythiaside, a constituent of the fruits of forsythia suspense, ameliorates scopolamine-induced memory impairment in mice. Biomol Ther, 2009a; 17(3):249–55. CrossRef
Kim S, Min-Soo K, Kwanghoon P, Hyeon-Joong K, Seok-Won J, Seung-Yeol N, Han JS, Chung CH. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res, 2016b; 40:55–61. CrossRef
Kinney JW, Shane MB, Andrew SM, Amanda ML, Arnold MS, Bruce TL. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer's Dement Transl Res Clin Interv, 2018; 4:575–90. CrossRef
Kumar S, Abhay KP. Chemistry and biological activities of flavonoids: an overview. Sci World J, 2013; 2013:1–16. CrossRef
Kunle OF, Egharevba HO, Ahmadu PO. Standardization of herbal medicines-a review. Int J Biodivers Conserv, 2012; 4(3):101–12. CrossRef
Kwon SH, Kim HC, Lee SY, Jang CG. Loganin improves learning and memory impairments induced by scopolamine in mice. Eur J Pharmacol, 2009; 619(1–3):44–9. CrossRef
Lautie E, Olivier R, Pierre D, Jean AB, Salvatore S. Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol, 2020; 11(4):1–37. CrossRef
Lee GY, Lee C, Park GH, Jang JH. Amelioration of scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evid Based Complement Alternat Med, 2017; 2017:1–9. CrossRef
Lee KY, Jeong EJ, Lee HS, Kim YC. Acteoside of callicarpa dichotoma attenuates scopolamine-induced memory impairments. Biol Pharm Bull, 2006; 29(1):71–4. CrossRef
Lee S, Kim J, Seo SG, Choi BR, Han JS, Lee KW, Kim J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res, 2014; 85:23–32. CrossRef
Lee Y, J Kim J, Jang S, Oh S. Administration of phytoceramide enhances memory and upregulates the expression of pCREB and BDNF in hippocampus of mice. Biomol Ther, 2013; 21(3):229–33. CrossRef
Li W, Sun L, Xiao S. Prevalence, incidence, influence factors, and cognitive characteristics of amnestic mild cognitive impairment among older adult: a 1-year follow-up study in china. Front Psychiatry, 2020; 11(2):1–9. CrossRef
Liu H, Junjian Z. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int J Neurosci, 2012; 122(9):494–9. CrossRef
Liu J, Yu H, Ning X. Effect of quercetin on chronic enhancement of spatial learning and memory of mice. Sci China C Life Sci, 2006; 49(6):583–90. CrossRef
Lu C, Yan W, Teng X, Qi L, Donghui W, Lijing Z, Fan B, Wang F, Liu X. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling. Front Pharmacol, 2018; 9:1–11. CrossRef
Mansouri MT, Farbood Y, Naghizadeh B, Shabani S, Mirshekar MA, Sarkaki A. Beneficial effects of ellagic acid against animal models of scopolamine- and diazepam-induced cognitive impairments. Pharm Biol, 2016; 54(10):1947–53. CrossRef
Mehta M, Abdu A, Marwan S. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int J Alzheimers Dis, 2012; 2012:1–8. CrossRef
Moss DE. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: are irreversible inhibitors the future? Int J Mol Sci, 2020; 21(3438):1–18. CrossRef
Mufson EJ, Binder L, Counts SE, DeKosky ST, deToledo-Morrell L, Ginsberg SD, Ikonomovic MD, Perez SE, Scheff SW. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol, 2012; 123(1):13–30. CrossRef
Ohta H, Kinzo M, Hiroshi W, Mineo S. Involvement of β1 but not β2-adrenergic systems in the antagonizing effect of paeoniflorin on scopolamine-induced deficit in radial maze performance in rats. Jpn J Pharmacol, 1993; 62(2):199–202. CrossRef
Oskouei Z, Soghra M, Fatemeh K, Hossein H. Evaluation of the effect of thymoquinone in d-galactose-induced memory impairments in rats: role of MAPK, oxidative stress, and neuroinflammation pathways and telomere length. Phytother Res, 2020; 35(4): 2252-2266. CrossRef
Oxford AE, Erica SS, Troy TR. Clinical trials in Alzheimer's disease: a hurdle in the path of remedy. Int J Alzheimers Dis, 2020; 2020:1–13. CrossRef
Park DH, Park SJ, Kim JM, Jung WY, Ryu JH. Subchronic administration of rosmarinic acid, a natural prolyl oligopeptidase inhibitor, enhances cognitive performances. Fitoterapia, 2010; 81(6):644–8. CrossRef
Park SJ, Ahn YJ, Oh SR, Lee Y, Kwon G, Woo H, Lee HE, Jang DS, Jung JW, Ryu JH. Amyrin attenuates scopolamine-induced cognitive impairment in mice. Biol Pharm Bull, 2014; 37(7):1207–13. CrossRef
Park SJ, Kim DH, Jung JM, Kim JM, Cai M, Liu X, Hong JG, Lee CH, Lee KR, Ryu JH. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur J Pharmacol, 2012; 676(1–3):64–70. CrossRef
Parle M, Dinesh D. Ascorbic acid: a promising memory-enhancer in mice. J Pharm Sci, 2003; 93(2):129–35. CrossRef
Peng WH, Hsieh MT, Wu CR. Effect of long-term administration of berberine on scopolamine-induced amnesia in rats. Jpn J Pharmacol, 1997; 74(3):261–6. CrossRef
Prashar Y, Gill NS, Kakkar S. A review on medicinal plants affecting amnesia on scopolamine induced model. PharmaTutor Mag, 2014; 2(12):20–8.
Rahimi R, Ghiasi S, Azimi H, Fakhari S, Abdollahi M. A review of the herbal phosphodiesterase inhibitors; future perspective of new drugs. Cytokine, 2010; 49(2):123–9. CrossRef
Ramsay RR, Popovic-Nikolic MR, Nikolic K, Uliassi E, Bolognesi ML. A perspective on multi-target drug discovery and design for complex diseases. Clin Transl Med, 2018; 7(1):1–14. CrossRef
Ratheesh G, Lingling T, Jayarama RV, Hariharan E, Asif S, Tai-Ping F, Ramakrishna S. Role of medicinal plants in neurodegenerative diseases. Biomanuf Rev, 2017; 2(2):1–6. CrossRef
Richardson C, Stephan BCM, Robinson L, Brayne C, Matthews FE, Cognitive Function and Ageing Study Collaboration. Two-decade change in prevalence of cognitive impairment in the UK. Eur J Epidemiol, 2019; 34(11):1085–92. CrossRef
Rizzi L, Idiane R, Matheus R. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int, 2014; 2014:1–8. CrossRef
Robertson LT. Memory and the brain. J Dent Educ, 2002; 66(1):30–42. CrossRef
Saroj K, Tulika S. Docosahexaenoic acid administration ameliorates scopolamine-induced memory impairment in mice. Asian J Pharm Clin Res, 2018; 11(6):349–52. CrossRef
Sharma D, Puri M, Tiwary AK, Singh N, Jaggi AS. Antiamnesic effect of stevioside in scopolamine-treated rats. Indian J Pharmacol, 2010; 42(3):164–7. CrossRef
Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (review). Mol Med Rep, 2019; 20(2):1479–87. CrossRef
Shi J, Liu Q, Wang Y, Luo G. Coadministration of huperzine a and ligustrazine phosphate effectively reverses scopolamine-induced amnesia in rats. Pharmacol Biochem Behav, 2010; 96(4):449–53. CrossRef
Stanciu GD, Luca A, Rusu RN, Bild V, Beschea Chiriac SI, Solcan C, Bild W, Ababei DC. Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules, 2019; 10(1):1–21. CrossRef
Stuve O, Weideman RA, Danni MM, David AJ, Bertis BL. Diclofenac reduces the risk of Alzheimer’s disease: a pilot analysis of NSAIDs in two us veteran populations. Ther Adv Neurol Disord, 2020; 13:1–11. CrossRef
Sun Q, Xie N, Tang B, Li R, Shen Y. Alzheimer’s disease: from genetic variants to the distinct pathological mechanisms. Front Mol Neurosci, 2017; 10:1–14. CrossRef
Tao L, Jianmei X, Yuting W, Shi W, Shuangchan W, Qiman W, Ding H. Protective effects of aloe-emodin on scopolamine-induced memory impairment in mice and H2O2-induced cytotoxicity in PC12 cells. Bioorg Med Chem Lett, 2014; 24(23):5385–9. CrossRef
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine, 2019; 14:5541–54. CrossRef
Tolar M, Susan A, Marwan S. The path forward in alzheimer's disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimer Dement, 2020; 16:1553–60. CrossRef
Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis, 2017; 57(4):1105–21. CrossRef
Wang B, Zhong Y, Gao C, and Li J. Myricetin ameliorates Scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem Biophys Res Commun, 2017; 490(2):336–42. CrossRef
Wang CM, Liu MY, Wang F, Wei MJ, Wang S, Wu CF, Yang JY. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol Biochem Behav, 2013; 106:57–67. CrossRef
Wang G, Chen L, Pan X, Chen J, Wang L, Wang W, Cheng R, Wu F, Feng X, Yu Y, Zhang HT, O'Donnell JM, Xu Y. The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget, 2016; 7(14):17380–92. CrossRef
Wang H, Wang H, Cheng H, Che Z. Ameliorating effect of luteolin on memory impairment in an Alzheimer’s disease model. Mol Med Rep, 2016; 13(5):4215–20. CrossRef
Xie Y, Zhao QY, Li HY, Zhou X, Liu Y, Zhang H. Curcumin ameliorates cognitive deficits heavy ion irradiation-induced learning and memory deficits through enhancing of Nrf2 antioxidant signaling pathways. Pharmacol Biochem Behav, 2014; 126:181–6. CrossRef
Yaari R, Ann H. Alzheimer’s disease clinical trials: past failures and future opportunities. Clin Trial Perspect, 2015; 5(3):297–309. CrossRef
Yunqi X, Junqiang Y, Peng Z, Li J, Gao H, Xia Y, Wang Q. Neurotransmitter receptors and cognitive dysfunction in Alzheimer’s disease and Parkinson’s disease. Prog Neurobiol, 2013; 97(1):1–13. CrossRef
Zhang L, Zhang Z, Fu Y, Yang P, Qin Z, Chen Y, Xu Y. Trans-cinnamaldehyde improves memory impairment by blocking microglial activation through the destabilization of iNos mRNA in mice challenged with lipopolysaccharide. Neuropharmacology, 2016; 110:503–18. CrossRef
Zhu Z, Yan J, Jiang W, Yao XG, Chen J, Chen L, Li C, Hu L, Jiang H, Shen X. Arctigenin effectively ameliorates memory impairment in Alzheimer’s disease model mice targeting both β-amyloid production and clearance. J Neurosci, 2013; 33(32):13138–49. CrossRef
GRAPHICAL ABSTRACT
 | [Click here to view] |