INTRODUCTION
Salbutamol sulfate (SS), chemically known as (RS)-1-(4-hydroxy-3-hydroxy-methyl phenyl)-2-(tert-butylamino)ethanol sulfate, is formulated as a 1:1 sulfate salt. It is the most extensively used sympathomimetic, indicating prominent bronchodilator and less cardiovascular impacts. It mitigates the congestion related to asthma, chronic obstructive pulmonary disease (COPD), and other forms of allergic airways disease (The Merk Index, 2006). The medication is additionally utilized for the anticipation of premature labor and exercise-evoked bronchospasm. Pharmacological action is a result of adenylyl cyclase stimulation, thus increasing the intracellular cyclic-adenosine monophosphate levels, promoting relaxation of the smooth muscles of bronchi and the uterus (Springhouse, 2008).
The drug is official in European Pharmacopoeia, British Pharmacopoeia, and Indian Pharmacopoeia. Different methods of detection of SS have been accounted for, either alone or in combination with other drugs, using chromatographic analytical techniques, like high-performance liquid chromatography (Walode et al., 2013), liquid chromatography–tandem mass spectrometry (Zhou et al., 2015), gas chromatography (Srichana et al., 1998), and by other nonchromatographic procedures (Basavaiah et al., 2006; Basavaiah et al., 2007). Various other principles for its detection are also reported (Muralidharan and Kumar, 2012)
Ipratropium bromide (IPB) is a congener of atropine obtained by treating atropine with isopropyl bromide, chemically described as 8-azoniabicyclo [3.2.1] octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl 8-(1-methyl ethyl) bromide (Abdine et al., 2003a). It was designed as an orally active, exceptionally polar drug which inadequately infiltrates the blood–brain barrier, thus preventing central side effects, while the sole significant side effect is dry mouth, at dosages higher than the therapeutic dose. IPB is a white to off-white crystalline substance freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in lipophilic solvents (Abdine et al., 2003b). The drug is used by inhalation, in the treatment of COPD and allergic rhinitis. It blocks all subtypes of muscarinic receptors, resulting in a decrease in the formation of cyclic guanosine monophosphate (c-GMP) that effectively averts the increase in intracellular concentration of calcium, thereby improving the contractility of smooth muscles and restraining mucus secretion (Mario et al., 2012).
IPB is devoid of structural conjugation (Abdine et al., 2003c); therefore, no analytical methods have been reported by the conventional UV method. The official methods for estimating IPB were the titrimetric method with silver nitrate for the assay of pure substance; BP (1998) used an high performance liquid chromatography (HPLC) method for the assay in pressurized inhalation, while the European, Japanese and American pharmacopoeia described other official methods. Hassan (2000) extended the application of derivative spectroscopy for its estimation. HPLC techniques were reported to quantify IPB in aerosol, liquid for nebulization (Nayak et al., 1992), and its related substances (Simms et al., 1998). Other methods reported are a radioreceptor assay method (Ensing et al., 1988) and a nonaqueous titration method. The detailed spectrophotometric methods depended on the oxidation of IPB with alkaline KMnO4 and complexation with iodine and bromocresol green (Hassan, 2000).
Not many methods have been accounted for in the simultaneous estimation of SS and IPB by HPLC. Most of the reported methods for their quantification depicted limitations (Jyothi et al., 2012; Kasawar and Farooqui, 2010; Nagaraju and Appaji, 2014; Vankudoth et al., 2013).
These bronchodilators are usually available in the market, either as pressured metered-dose inhalers, dry powder inhalers, soft mist inhalers, or as nebulization solutions. Challenges experienced by incorrect inhaler device usage bringing about befuddled COPD patients prompts suboptimal drug administration, thus rendering poor adherence to medical care (Braido et al., 2016). The alarming rise in mortality and morbidity is caused by the progressive nonreversible disease COPD and the potential drawbacks of the inhaler therapy used in the management of COPD; the economic burden experienced by patients due to hospitalization and exacerbation (Kallaru et al., 2015) happening due to nonadherence of therapy can be resolved by application of the novel drug delivery systems, such as the transdermal drug delivery system which offers numerous advantages, such as increased bioavailability, decreased frequency of dosing, capacity to entrap both hydrophilic and lipophilic compounds, reduced toxicity, and easy termination of medication by removal of the formulation (Ali et al., 2015).
Precise evaluation of the drugs trapped in the matrix is vital, since it is the integral factor that can be related to its therapeutic effectiveness. Hence, we felt the requirement to develop a validated analytical method for the estimation of SS and IPB and to quantify the in-vitro drug release from the in-house manufactured transdermal patch.
EXPERIMENTAL STUDY
Materials
HPLC grade acetonitrile and methanol were obtained from Fisher Scientific (Mumbai, India), IPB (CAS No-18559-94-9) was acquired from Vamsi Laboratories, Sholapur, India, and SS (CAS No-60205-81-4) was gifted by Elegant Drugs, India, and was used as supplied. Evonik Degussa India Pvt. Ltd., Mumbai, was benevolent enough to gift samples of Eudragit Polymer Eudragit RL 100 (RLPO) and Eudragit RSPO. The plasticizer triethyl citrate and mercury were bought from S.D. Fine Chemicals Ltd., Mumbai. Different reagents used were of analytical quality.
METHODS
Instrumentation
The chromatographic framework comprised the Shimadzu Prominence HPLC system (Kyoto, Japan) equipped with a pump (LC-20AD), photo diode array detector (SPD-M20A), an online degasser (DGU-20A5), an autosampler (SIL-20AC HT), a rheodyne injection valve with a 20-μl loop and a column oven (CTO-10ASVP). Data acquisition was rendered using the Shimadzu LC software solution (version 1.25).
Chromatographic conditions
A Luna C18(2) column (150 × 4.6 mm i.d., 5-μm particle size; Phenomenex, CA, USA) fitted with a security guard column (C18 ODS; 4 × 3.0 mm i.d.; Phenomenex, CA, USA) was utilized for the chromatographic separation of SS and IPB. The mobile phase injected at a flow rate of 1 ml/minute involved a blend of acetonitrile:phosphate buffer (pH adjusted to 3.0 ± 0.5 with o-phosphoric acid) in 30:70 v/v ratio. The column temperature was 30°C and the detection wavelength was 212 nm. The mobile phase was filtered and degassed prior to use. The chromatographic run injecting 10-μl volume was stopped at the end of 6 minutes. Before the injection of standards and samples, the column was equilibrized with the mobile phase for 60 minutes. Stock solutions of 1,000 μg/ml of SS and IPB were prepared using the mobile phase and necessary dilutions were made to acquire concentrations of 2–12 μg/ml.
Validation studies
The developed HPLC method was validated in accordance with the guidelines of the International Council for Harmonization (ICH) (International Conference On Harmonization, 2005) for various parameters, such as the system suitability, precision, linearity, accuracy, specificity, robustness, limit of quantification (LOQ) and detection (LOD). System suitability tests were carried out by injecting six replicates of standard SS and IPB solutions and determining the tailing factor, number of theoretical plates, and percentage of relative standard deviation (%RSD) of retention time and peak area. Linearity was measured by using the least squares regression model to assess the slope, y-intercept, and correlation coefficient. The calibration curves were obtained from various SS and IPB concentrations ranging from 2 to 12 μg/ml and were tested in triplicates. Precision was evaluated by determining the repeatability of three different standard solutions for six times on the same day (intraday) and examining the same three standard arrangements three times on three successive days (interday). Accuracy was tested at three diverse concentration levels by spiking the pre-analyzed commercially available samples with known concentrations of SS and IPB and later deciding the %RSD. Specificity was studied by checking the blank patches chromatograms amid in-vitro release to check for potential interference by the excipients added. The LOD and LOQ values were decided numerically based on the standard deviation of the response and the slope. They were expressed as LOQ=3.3 σ÷S and LOQ=10σ÷S. The robustness of the developed reverse phase high performance liquid chromatography (RP-HPLC) method was evaluated by deliberate changes to the optimized chromatographic conditions based on the %RSD values of the system suitability parameters, such as change of ± 0.2 ml/minutes, ± 2%, ±5°C, and ± 2 nm in the flow rate, mobile phase composition, column temperature, and wavelength, respectively.
Fabrication of patches
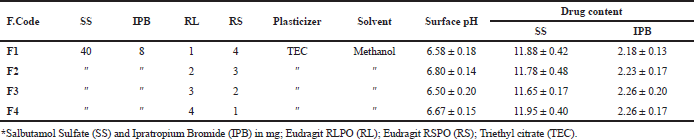
Four films coded F1, F2, F3, and F4 were prepared according to the composition stated in Table 6, by dissolving the drugs to the previously prepared methanolic polymeric solution comprising plasticizer, sonicated to remove entrapped air bubbles, and the final mixture was made to 10 ml. Drug-loaded polymer solutions were casted onto a flat-bottomed Anumbra petri dish using mercury as a substrate and dried overnight at room temperature (Parhi and Suresh, 2016).
Formulation considerations
The total dose (Eriksen and Robinson, 2006) for once-daily controlled-release formulation of SS and IPB was calculated using the available pharmacokinetic data as per Robinson Eriksen’s equation. The loading dose for incorporation into the formulation was calculated considering the radius 4.4 cm of the Anumbra molds (Parhi and Suresh, 2016).
In-mano evaluation
The prepared films were graded either as transparent or translucent based on the opacity of the film, their texture was given two grades (smooth/rough), and finally, the nature of the film was noted as either flexible or brittle. The patches which could withstand in-mano stress to maintain its structural integrity and the film which could be rolled and folded were termed as tensile and pliable, respectively (Ham et al., 2013). A study of tack properties enabled us to understand the adhesive nature of the film. It was studied a week after the preparation of the transdermal patches, by pressing the thumb lightly on a patch for about 5 seconds and then quickly withdrawing. The folding endurance was measured manually by subjecting a small strip of the film (2 cm × 2 cm) to repeated folds in the same place. The number of times the film was folded without developing visible cracks or breaking was noted (Shailesh et al., 2011).
Surface pH
The surface pH of the patches was determined by Bottenberg et al.’s method and reported in Table 6. The surface pH of a 1-cm2 patch, previously soaked in 0.5 ml double-distilled water for 1 hour, was noted by bringing a glass electrode close to the surface of the patch and permitting it to equilibrate for 1 minute (Bharkatiya et al., 2010).
Drug content
Patches of 1-cm2 area, placed in tubes containing 10-ml mobile phase, were shaken with the help of a rotary flask shaker for 24 hours at room temperature, till the complete dissolution of the drugs. After prior filtration, 0.5 ml drug aliquots was pipetted out into vials containing 1.5 ml of mobile phase, and each film formulation was tested in triplicate and the results are expressed as the mean and standard deviation in Table 6 (Vijaya et al., 2012).
In-vitro drug release studies
A Franz diffusion system having six vertical 12.5-ml capacity receptor cells with an effective diffusion area of 1.77 cm2 and a PermeGear diffusion system (Perme Gear, Inc. Hellertown, PA) filled with phosphate buffer pH 7.4 were used as the diffusion medium. A magnetic stirrer bar of 10 × 2.5 mm served for the continuously stirring the receptor solution maintained at 37°C ± 0.5°C. The dialysis membrane of pore size 0.45 μ, soaked in phosphate buffer overnight, was mounted between the donor and receptor compartments so that it just touched the receptor fluid’s surface. The prepared transdermal film was placed on the dialysis membrane and covered with aluminum foil, 0.5 ml sample aliquots diluted to 2 ml was withdrawn every 2 hours (for 24 hours), and the released drug was quantified by detecting using the HPLC at 212 nm. The drug release rate is estimated and shown in Figure 3 and 4.
Analysis of commercial formulations
The application of the method in estimating drug content from marketed formulation was tested by appropriate dilution of capsules and the results are shown in Table 4. The typical chromatograms of a pure, commercial formulation and in-vitro release pattern are shown in Figure 2.
RESULTS AND DISCUSSION
Analytical method development
Absence of conventional method
The concern in evaluating the stated drugs simultaneously by the simple analytical technique UV–visible spectrophotometry was confirmed by the trials of the simultaneous equation method and the derivative spectroscopic method. The low absorbing nature of IPB and almost similar zero-crossing points made their simultaneous estimation tedious. Subsequently, our aim was to build another method of analysis for the estimation of these drugs.
Optimization of chromatographic conditions
The RP-HPLC method was optimized by changing different parameters, such as mobile phase composition, flow rate, detection wavelength, and column oven temperature, with the true objective to acquire sharp and symmetrical peaks with excellent baseline separation. The Luna C18(2) column (150 × 4.6 mm i.d., 5 μm) was used as the stationary phase and the detection wavelength was fixed at 212 nm due to enhanced sensitivity. No drug elution was observed during the trials comprising methanol–water, methanol–acetonitrile, methanol–potassium and dihydrogen phosphate buffer in various proportions, but when methanol is replaced by acetonitrile, peaks with shorter retention time were eluted. Therefore, further trials were carried out by altering the proportions of acetonitrile and buffer. The ACN:PBS (30:70 v/v) ratio was finalized and gave well-resolved peaks with no significant improvement in peak shape by additional trials. Mobile phase was also considered as a diluent to avoid disturbances in the baseline. The temperature was set at 30°C and the flow rate at 1.0 ml/minute, thus resulting in a well-resolved peak with better shape, shorter retention time, and lesser tailing factor.
METHOD VALIDATION
Analytical method validation is carried out to guarantee that the developed method is reliable and adequate for its intended purpose. The proposed method was validated in accordance with the ICH guidelines by reviewing parameters of system suitability, specificity, linearity, accuracy, precision, LOD, LOQ, and robustness.
System suitability
System suitability test guarantees that the set chromatographic conditions ensure proper separation, and the parameters are abridged and presented in Table 1. The high number of theoretical plates (n > 2,000) exhibited good column efficiency and the tailing factor values (T<2) indicated good peak symmetry. The %RSD of the retention time and peak area was within 1%, indicating the chromatographic setup’s reproducibility and repeatability (%RSD<1). The resolution (Rs) of 4.8 between SS and IPB indicated a high degree of peak separation (Rs>2.0). All tested parameters for further validation and sample analysis were within acceptable limits, suggesting the suitability of the chromatographic arrangement.
Specificity
The retention times of SS and IPB were 2.4 and 3.8 minutes, respectively. No peaks were eluted when the mobile phase alone or blank patches were exposed to similar experimental conditions as that of drug-loaded films, while the drug-loaded patches and the commercial formulations showed distinct peaks as that of pure drugs, thus indicating no interference from the formulation ingredients. Chromatograms depicting specificity is shown in Figure 1.
Linearity, detection, and quantification limits
The calibration curves of SS and IPB were linear over the tested concentration range of 2–12 μg/ml and the coefficient of correlation for both bronchodilators was higher than 0.999, proposing an excellent correlation and good linearity for the method developed is presented in Table 1. The LOD values were found to be 0.42 μg/ml for SS and 0.44 μg/ml for IPB, while the LOQ values were found to be 1.26 μg/ml for SS and 1.34 μg/ml for IPB, indicating a sufficiently sensitive method to determine the drug even from low permeable matrix systems.
Precision
Intraday and interday precision results are expressed in %RSD in Table 2 and in both cases were found to be below 2%, demonstrating that the developed method is precise.
Accuracy
Table 3 summarizes the mean %recoveries, indicating the low variability and closeness between experimental and true values.
Robustness
Table 5 depicts the robustness data with %RSD values (<1) and system suitability parameters (theoretical plate, n > 2,000; tailing factor, T<2). The parameter tested was found to be satisfactory, and hence confirms that the developed method is robust.
Characterization of transdermal systems and applicability of HPLC method
Consideration of formulation parameters
SS of 5 mg and IPB of 1 mg per patch were found as the optimum dose. Our aim was to prepare an 8-cm2 patch while the area of the anumbra plate was found to be 60.7904 cm2. Hence, the amount of the drug required was approximately 40 mg of SS and 8 mg of IPB.
 | Table 1. System suitability parameters of SS and ipratropium bromide (8 μg/ml). [Click here to view] |
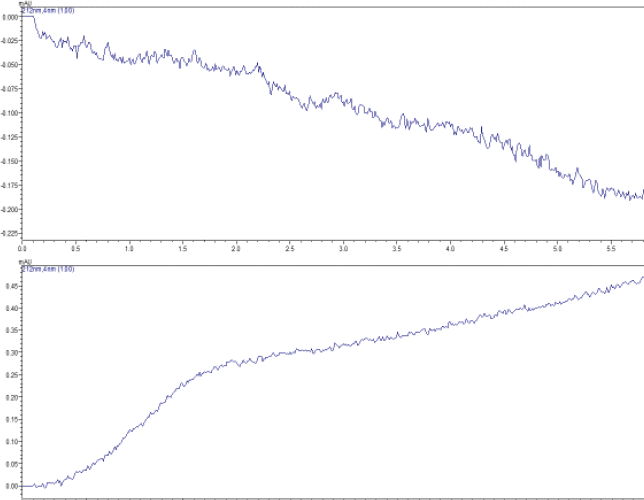 | Figure 1. Typical chromatogram showing blank mobile phase (d) and release from the blank patch (e). [Click here to view] |
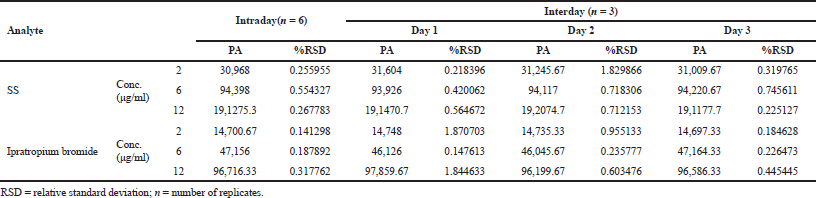 | Table 2. Precision data of SS and ipratropium bromide. [Click here to view] |
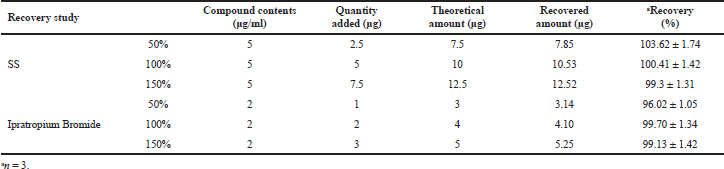 | Table 3. Recovery studies of SS and ipratropium bromide. [Click here to view] |
In-mano evaluation
All formulations appeared to be as transparent, flexible, tensile, pliable, and have a smooth texture. All the stated characteristics were more vivid with the increasing content of RL. Hence, the developed films are said to possess the prerequisites for an optimized patch. The tackiness of the films was in the following order F1>F2>F3>F4. This pattern can be correlated with the direct proportionality between tackiness and RS content. The effect of the plasticizer, polymer, and solvent is ruled out, since these factors were unaltered in these formulations. The films exhibited no signs of folds even after 100 folds, specifying the ability of the films to be unaffected when it experiences the natural fold of the skin.
 | Table 4. Assay of commercial formulation containing SS and ipratropium bromide. [Click here to view] |
Surface pH
The surface pH of all the formulations is presented in Table 6, and the results indicate the compatibility of the prepared patch with the skin.
Drug content
Drug content analysis was carried out for all the prepared transdermal systems and the results displayed in Table 6 show good uniformity and a higher degree of reproducibility with a low standard deviation. The drug content from the marketed formulation is presented in Table 4.
In-vitro drug release studies
The developed RP-HPLC method was also extended to quantify the drug release pattern of the in-house manufactured patches and the in-vitro release profile of SS and IPB from formulations is graphically shown in Figures 3 and 4, respectively.
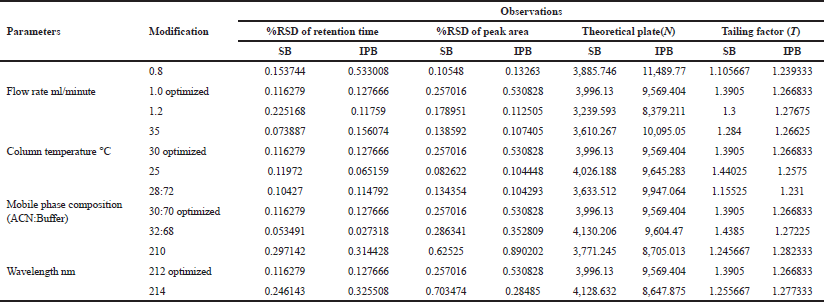 | Table 5. Robustness data of salbutamol sulphate and ipratropium bromide. [Click here to view] |
 | Table 6. Evaluation of the films incorporating SS and ipratropium bromide [Click here to view] |
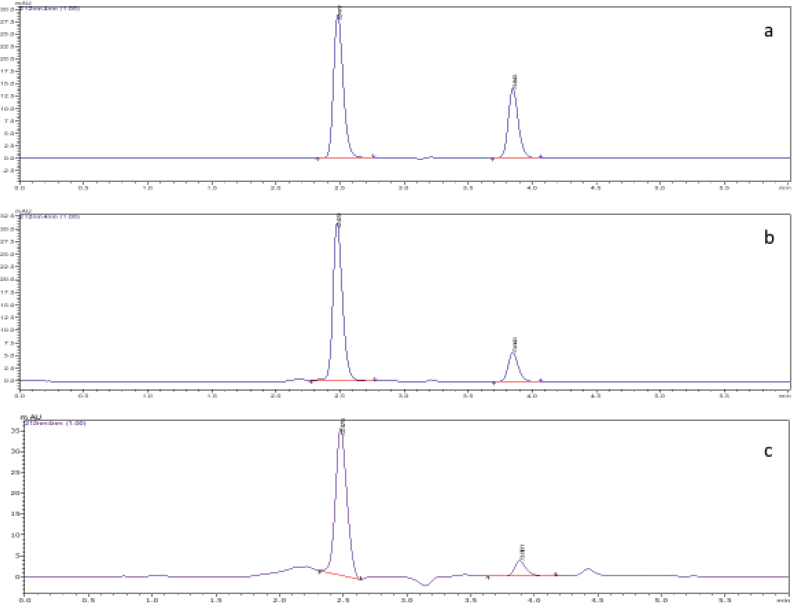 | Figure 2. Typical chromatogram showing the peaks of salbuatmol sulfate and ipratropium bromide. (a: pure drugs; b: commercial samples c: release from patch). [Click here to view] |
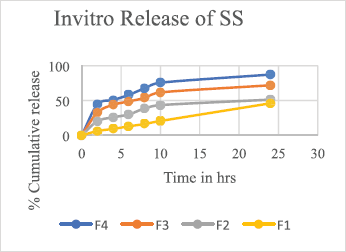 | Figure 3. Graph depicting the in vitro release studies of salbutamol sulphate from formulations and F1–F4 transdermal patches using a dialysis membrane. [Click here to view] |
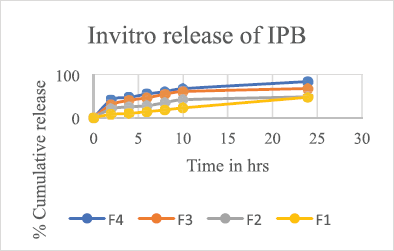 | Figure 4. Graph depicting the in-vitro release studies of ipratropium bromide from formulations and F1–F4 transdermal patches using a dialysis membrane. [Click here to view] |
CONCLUSION
In conclusion, the strategically developed, ICH-validated RP-HPLC method for successful quantification of SS and IPB content in pure and marketed formulation is hereby stated. The validation parameters are in accordance with the ICH guidelines. This method adequately helps to quantify drugs released from in-house manufactured transdermal patches at regular intervals and was found to be more economical, namely factors showing decreased elution time (6 minutes), flow rate (1 ml/minute), and consumption of costly solvents.
ACKNOWLEDGMENTS
The authors acknowledge KAHER, Belagavi, for funding the project and providing all research facilities. The authors appreciatively acknowledge Elegant Drugs, Evonik Degussa, India, for providing the gift samples of SS and polymers RLPO and RSPO, respectively. Support extended by Dr. Malleswara Rao Peram and Mr. Satveer Jagwani is also acknowledged.
CONFLICT OF INTEREST
Authors declared that they do not have any conflicts of interest.
REFERENCES
Abdine H, Belal H F, Al-Badr AA. Ipratropium bromide. Profiles Drug Subst Excip Relat Methodol, 2003a; 30:59–83.
Abdine H, Belal HF, Al-Badr AA. Ipratropium bromide: methods of chemical and biochemical synthesis. Profiles Drug Subst Excip Relat Methodol, 2003b; 30:85–99. CrossRef
Abdine H, Belal HF, Al-Badr AA. Ipratropium bromide: analytical methods. Profiles Drug Subst Excip Relat Methodol, 2003c; 30:101–16. CrossRef
Ali S, Shabbir M, Shahid N. The structure of skin and transdermal drug delivery system – a Review. Res J Pharm Technol, 2015; 8(2):103–9. CrossRef
Basavaiah K, Somashekar BC, Ramakrishna V. Titrimetric and spectrophotometric determination of salbutamol sulphate in pharmaceuticals using chloramine-T and two dyes. Anal Chem Ind J, 2006; 2(5):179–86.
Basavaiah K, Somashekar BC, Ramakrishna V. Rapid titrimetric and spectrophotometric methods for salbutamol sulphate in pharmaceuticals using N-bromosuccinimide. Acta Pharm, 2007; 57(1):87–98. CrossRef
Bharkatiya M, Nema RK, Bhatnagar M. Designing and characterization of drug free patches for transdermal application. Int J Pharm Sci Drug Res, 2010; 2(1):35–9.
British Pharmacopoeia. Vol II, Her Majesty’s Stationary Office, London, UK, p 740, 1761, 1998.
Braido F, Chrystyn H, Baiardini I, Bosnic-Anticevich S, Van Der Molen T, Dandurand RJ. “Trying, but failing” – the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract, 2016; 4(5):823–32. CrossRef
Ensing K, Pol M, De Zeeuw Ra. Radioreceptor assays of ipratropium bromide in plasma and urine. J Pharm Biomed Anal, 1988; 6(5):433–9. CrossRef
Eriksen SP, Robinson Jr. Theoretical formulation of sustained-release dosage forms. J Pharm Sci, 2006; 55(11):1254–63. CrossRef
Hassan EM. Determination of ipratropium bromide in vials using kinetic and first-derivative spectrophotometric methods. J Pharm Biomed Anal, 2000a; 21(6):1183–9. CrossRef
Hassan EM. Spectrophotometric determination of ipratropium bromide in liquid for nebulization. Anal Lett, 2000b; 33(8):1531–43. CrossRef
Ham As, Lustig W, Yang L, Boczar A, Buckheit Kw, Buckheit Rw. In vitro and ex vivo evaluations on transdermal delivery of the hiv inhibitor Iqp-0410. PloS One, 2013; 8(9):1–11. CrossRef
International Conference On Harmonization (ICH). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Validation of analytical procedures: text and methodology, Q2(R1). International Conference On Harmonization (ICH), Geneva, Switzerland, 2005.
Jyothi, N, Gopal, KV, Seshagiri Rao, JVLN. Development and validation of an HPLC method for the simultaneous estimation of the salbutamol sulphate and ipratropium in inhalation dosage forms. Int J Pharm Sci, 2012; 2(4):79–83.
Kasawar GB, Farooqui M. Development and validation of a stability indicating RP-HPLC method for the simultaneous determination of related substances of albuterol sulfate and ipratropium bromide in nasal solution. J Pharm Biomed Anal, 2010; 52(1):19–29. CrossRef
Kallaru H, Nagasubramanian Vr, Balakrishnan Hp, Gopal K, Palani T. Impact of severity of the disease on cost of illness and quality of life of patients with chronic obstructive pulmonary disease. J Young Pharm, 2015;7(2):106–12. CrossRef
Mario C, Clive PP, Luigino CM. Gabriella MM. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev, 2012; 64(3):450–504. CrossRef
Muralidharan S, Kumar J. High performance liquid chromatographic method development and its validation for Salbutamol. J Pharm Res Int, 2012; 2(4):228–37. CrossRef
Nayak VG, Bhate VR, Dhumal SN, Nerurkar VR, Purandare SM, Dikshit PM, Gaitonde CD. Estimation of ipratropium bromide from aerosols by reversed-phase liquid chromatography. Drug Dev Ind Pharm, 1992; 18(15):1681–9. CrossRef
Nagaraju, P, Appaji, SCHVSS. Development and validation of novel RP-HPLC method for simultaneous estimation of levosalbutamol and ipratropium bromide in pharmaceutical dosage forms. Int J Res Pharm Chem, 2014; 4(3):628–35.
Parhi R, Suresh P. Formulation optimization and characterization of transdermal film of simvastatin by response surface methodology. Mater Sci Eng C, 2016; 58:331–41. CrossRef
Simms PJ, Towne RW, Gross CS, Miller RE. The separation of ipratropium bromide and its related compounds. J Pharm Biomed Anal, 1998; 17(4–5):841–9. CrossRef
Srichana T, Martin GP, Kicman A, Walker C, Marriott C. Determination of salbutamol in human plasma and urine by gas chromatography-mass spectrometry. J Pharm Pharmacol, 1998; 50(9):86–6. CrossRef
Shailesh TP, Charmi GP, Chhagan NP. Formulation and evaluation of transdermal patch of repaglinide. ISRN Pharm, 2011; 1–9. Article ID 651909. CrossRef
Springhouse. Clinical pharmacology made incredibly easy! 3rd edition, Lippincott Williams & Wilkins (LWW), Philadelphia, PA, 2008.
The Merk Index. An encyclopedia of chemicals, drugs and biological. 14th edition, Merk Laboratories, Kenilworth, NJ, p 216, 2006.
Vijaya R, Manjunath MN, Uma Maheswari S. Formulation and evaluation of transdermal films of anti-depressant Drug Amitriptyline Hydrochloride using Eudragit E100, HPC and PVP polymers. Mater Sci, 2012; 4(2):639–43.
Vankudoth R, Pratap R, Umashankar B, Sai Sneha G, Chawan S, Aparna A. RP-HPLC method for simultaneous estimation of Ipratropium bromide and Levosalbutamol in pharmaceutical metered dose inhalers. Int J Res Pharm Chem, 2013; 3(1):112–20.
Walode SG, Deshpande SD, Deshpande A V. Stability indicating RP-HPLC method for simultaneous estimation of salbutamol sulphate and guaifenesin. Der Pharm Sin, 2013;4(2):61–7.
Zhou T, Zeng J, Liu S, Zhao T, Wu J, Lai W, et al. Study on the determination and chiral inversion of R-salbutamol in human plasma and urine by liquid chromatography-tandem mass spectrometry. J Chromatogr B: Anal Technol Biomed Life Sci, 2015;1002:218–27. CrossRef