INTRODUCTION
Because of its high frequency and concomitant hazards of cardiovascular and kidney diseases, hypertension (HTN) is a major public health challenge globally. It was recognized as the leading mortality risk factor (Campbell et al., 2012). The incidence of HTN has been commonly recorded in different areas of the globe and the issues connected with HTN are rising more quickly in developing countries, such as Nepal. The study revealed that the prevalence of HTN in Nepal’s rural society and the urban area has increased threefold (Vaidya et al., 2012). The oral route is extensively utilized to deliver the therapeutic agents for the management of HTN due to the low treatment price and ease of administration, resulting in elevated patient compliance rates. Over 50% of the market’s drug delivery systems are oral drug delivery systems (Asija et al., 2015). The conventional drug delivery system achieves and holds the drug concentration within the optimum therapeutic range necessary for therapy, only if administered multiple times a day (Wen et al., 2015). This results in significant fluctuation in drug levels. Hence, to overcome these issues, several technical advancements have been made, such as novel drug delivery systems (NDDS) that can significantly improve the medication method and deliver several therapeutic advantages (Gao and Jiang, 2017). Despite the benefits of NDDS in terms of therapeutic considerations, there are several technical and biological challenges. For instance, modifying the GI transit time is a promising challenge for the formulation of an oral controlled drug delivery system (Ammar et al., 2016). Conventional drug delivery systems like tablets and capsules offer a particular concentration of drugs in the systemic circulation, lacking control over the rate of drug delivery, as well as triggering significant changes in the concentrations of plasma drugs. Moreover, there is a considerable fluctuation in the gastric emptying rate. Less time spent in the gut may result in inadequate drug absorption. Besides, the magnitude of absorption in the stomach or upper intestine is higher (Zhang et al., 2016). Hence, to figure the limitation of the single oral dosage form, the concept of a floating controlled drug delivery system was taken into account. Several types of control drug delivery systems have been discovered, out of which the concept of floating microencapsulation dosage form was found to be convincing in terms of its financial and technical prospects. Moreover, it was found to be more effective in addressing the several issues of conventional dosage form, especially concerning their therapeutic and safety issues (Bhardwaj et al., 2014; Farooq et al., 2017).
Microencapsulation is defined as “… a method by which solid, fluid, or gaseous active ingredients are packaged in a second product to protect the active ingredient from the surroundings.” (Hu et al., 2017) Microencapsulation is primarily known to convert the liquids into solids, by altering the surface and colloidal properties, offering a barrier for environmental protection and regulating the rate of release or availability features of coated materials. Macropackaging techniques can achieve several of these properties; however, the uniqueness of microencapsulation is the size and successive uses of the covered particles (Chatterjee et al., 2014). The study is based on the management of HTN with Enalapril Maleate (angiotensin-converting enzyme inhibitor) as a drug of choice that is 60% absorbed and undergoes de-esterification converting into an active metabolite enalaprilat, a potential angiotensin-converting enzyme inhibitor (Thabet et al., 2018). It is efficient against essential and reno-vascular HTN and cardiac congestion (Ipate et al., 2018; Swamy et al., 2013). Enalapril Maleate (EnM) microsphere increases bioavailability and compliance by decreasing the frequency of administration and helps maintain the consistency of plasma drug concentration during drug therapy (Ammar et al., 2016; Nila et al., 2014).
MATERIALS AND METHODS
EnM (an active pharmaceutical ingredient) was kindly gifted by Lomus Pharmaceutical, Nepal. Hydroxyl propyl methylcellulose (HPMC) was purchased from Kemphasol. Calcium chloride was purchased from Quligen Fine Chemicals. Dichloromethane (DCM) was purchased from Avantor Performance Ltd. Ethylcellulose (EC) and sodium alginate (Na-Alg) polymer, Tween 80, and other solvents such as ethyl alcohol and acetonitrile (ACN) were purchased from S.D. Fine Limited. All of the materials and solvents were of analytical grade.
Formulation of floating microsphere
The formulation of the EnM-loaded alcohol microsphere (Formulation-A) was carried out by a solvent evaporation method (Prajapati et al., 2012). Weighed quantity of EnM (5 mg) and a suitable amount of polymers (EC and HPMC) of varying proportions were dissolved in a mixture of varying proportions of DCM and ethanol. The resulting solution was dropwise introduced into the distilled water containing 0.6% Tween 80 as a surfactant. The entire solution was stirred at the agitation speed of 1,200 rpm for 45 minutes. After the complete evaporation of the volatile solvent, the emulsion was filtered and washed with distilled water (2–3 washes). The collected microspheres were air-dried and stored in desiccators. Similarly, another EnM-loaded ACN microsphere (Formulation-B) was formulated by using varying proportions of DCM and ACN as a solvent medium. The solubility of EnM was higher in ethanol and sparingly soluble in DCM and ACN. On the higher temperature, HPMC was soluble in water, while insoluble in DCM and ACN. EC was insoluble in water, sparingly soluble in DCM and ACN, but freely soluble in ethanol. The selection of the solvent medium was based on the earlier studies that confirm the use of solvents like DCM and ACN in various food and pharmaceutical industries (Amid et al., 2015). A comparison of Formulation-B was made with Formulation-A.
Optimization of formulation
The optimization of the EnM-loaded microsphere was carried out by using design expert 10 (State-Ease Inc., Minneapolis, MN). Three independent variables, that is, polymer-to-EnM ratio (A), alcohol-to-DCM ratio (B), and stirring time (C), were taken into account, and the effect of these independent variables was studied on the observed responses, that is, particle size, drug loading, and entrapment efficiency. The polymer-to-drug ratio used was in the ranges of 50:1, 100:1, and 150:1; similarly, the solvent ratio (DCM: ethanol) was in the ranges of 1:1, 2:1, and 3:1, and stirring time ranged between 20, 40, and 60 minutes. The optimization design is depicted in Table 1. With the 3 factors × 3 levels, 17 different runs were carried out and the response was observed for each run. The best optimized formulation was considered for further study.
CHARACTERIZATION
Particle size analysis
Optical microscopy was used for the determination of the particle size of the formulated microspheres. Optical microscope Olympus BX53 was used. Each batch containing more than 300 floating microspheres was placed on a clean slide, and the diameter of the particles was measured randomly. The eyepiece micrometer was calibrated with the help of a stage micrometer. Edmondson’s equation was used for the determination of the average particle size (Abbas et al., 2014):
where n = number of microsphere checked and d = mean size range.
Percentage yield
The percentage yield was estimated as the amount of microspheres obtained from the total amount of drugs and nonvolatile excipients deployed for the formulation of the microspheres (Gaur et al., 2014):
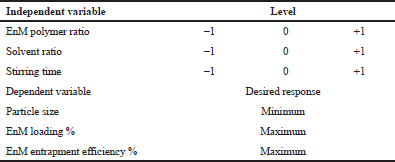 | Table 1: Optimization design including independent and dependent variables with their responses. [Click here to view] |
Drug loading and encapsulation efficiency
Accurately weighed microspheres (100 mg) were kept overnight in 0.1 N HCl for the extraction of the drug. Filtrates were diluted suitably and EnM was quantified spectrophotometrically using UV–Vis spectrophotometer at 207 nm. The percentage of drug loading was calculated as:
where L is the percentage drug loading of microspheres, Qm and Wm represent the quantity of drug and microsphere in gram, respectively (Farooq et al., 2017). The percentage of encapsulation was estimated as:
where E is the percentage encapsulation of microspheres, Qp and Qt represent the quantity of the drug encapsulated in microspheres and the theoretical drug content in gram, respectively (Garg et al., 2010; Senthikumar et al., 2010; Sharma et al., 2015).
Micrometric studies of microsphere
Bulk density
To determine the bulk density, suitably weighed microspheres were introduced into the measuring cylinder (100 ml) and the volume was observed, representing bulk quantity that involves real powder quantity and microspheres void space. Bulk density was estimated as the mass of microspheres divided by the volume of microspheres before tapping (Ramteke et al., 2015):
Tapped density
A suitable quantity of the microsphere was introduced into a measuring cylinder and was allowed to tap 100 times using the bulk density apparatus. The final tapped volume of the microsphere was recorded. The tapped density was estimated as the total mass of microspheres divided by the tapped volume of microsphere (Bhardwaj et al., 2014):
Carr’s (compressibility) index
For the powders with good flow characteristics, Carr’s index value usually lies below 15%, whereas Carr’s index above 25% indicates the poor flowability (Farooq et al., 2017).
Hausner’s ratio
Hausner’s ratio of microspheres was determined as the ratio of tapped density to the bulk density using the following equation (Agrawal et al., 2017; Beringhs et al., 2018):
Angle of repose
For the measurement of the resistance of the particle flow, the angle of repose (α) was estimated. The fixed funnel method was used for the determination of the angle of repose. The height of the funnel was adjusted accurately so that there was a narrow space between the tip of the funnel and the heap of the microsphere. Weighed microspheres were passed freely through the funnel on to the surface (Wang et al., 2010).
The height and radius of the powder were estimated, and the angle of repose was calculated as (Subham et al., 2010):
where α = angle of repose,
h = height, and
r = radius of a heap of microsphere
Test for buoyancy
The test for the buoyancy of the microspheres was studied in a USP type II dissolution test apparatus. A suitable amount of microspheres (100 mg) was dispersed in the 0.1 M HCl (300 ml) solution. Tween 80 (0.02%) as a surfactant was added to the solution. The medium was stirred with a paddle rotating at 100 rpm, and the temperature was adjusted at 37°C ± 0.5°C. After 12 hours, both the floating and the settled fractions of microspheres were amassed separately by filtration and were dried at 45°C until a constant weight was obtained (Patela et al., 2011):
In vitro drug release study
In vitro drug release studies were conducted in a USP type II dissolution apparatus. Accurately weighed floating microspheres (equivalent to 5 mg EnM) were filled into a capsule and introduced in a 900 ml dissolution medium comprising 0.1 N HCL. Tween 80 (0.02%) was added as a surfactant, which helps the solubilization of drugs in the dissolution medium. The medium was stirred with a paddle rotating at the speed of 100 rpm. Samples of 5 ml were withdrawn at 30 minutes, 1, 2, 4, 6, and up to 12 hours. The samples were analyzed by a UV spectrophotometer at 207 nm for the determination of the concentration of drugs present. 5 ml of fresh dissolution medium was introduced after each sampling to maintain the sink condition (Gupta et al., 2014; Tamizharasi et al., 2011). A similar dissolution study was carried out for the pure drug (5 mg) as well, which worked as a control. The comparative dissolution study was performed for Formulation-A and Formulation-B concerning control.
RELEASE KINETICS
For the determination of the release kinetics, the data obtained from the in vitro drug release study was fitted with various kinetics models (zero-order, first-order, Higuchi, Korsmeyer–Peppas) to understand the general mechanism of drug release from the microsphere. The coefficient of correlation values (R2) was evaluated for each release kinetic model and comparisons were made in between.
STATISTICAL ANALYSIS
The results of all the experiments were expressed as mean ± SD and were run in triplicate. GraphPad Prism version 7 software (Graph Pad Software Inc., La Jolla, CA) was used to carry out the statistical analysis. A two-way analysis of variance with Tukey’s post-hoc test was used to analyze the data. Statistical significance was predefined at p < 0.05.
RESULTS AND DISCUSSION
Statistical optimization by response surface methodology
For the optimization of the formulation, a “Box–Behnken experimental design” was considered using three independent variables at three different levels. The direct influence of the selected independent variables like drug-to-polymer ratio (A), solvent ratio (B), and stirring time (C) on dependent variables like particle size (R1), % drug loading (R2), and % entrapment efficiency (R3) were observed and the observed responses for 17 generated runs are depicted in Table 2.
The best fit model that was chosen for all the three dependent variables, generated by the design expert software 10 (State-Ease Inc., Minneapolis, MN), was the quadratic model with a coefficient of correlation (R2) close to 1. The summary of the model of regression analysis for responses R1, R2, and R3 and regression equation for a fitted quadratic model is depicted in Table 3.
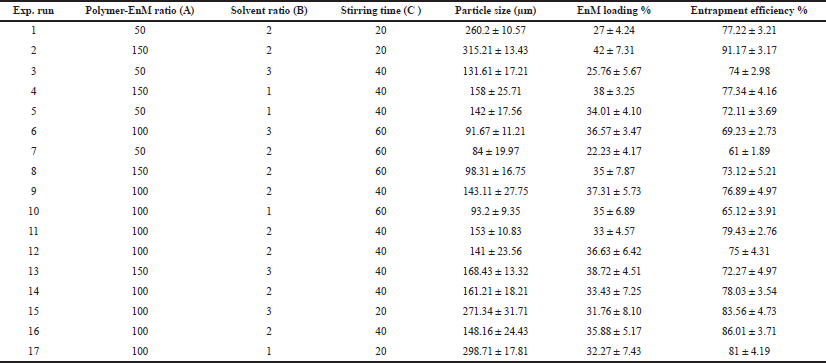 | Table 2: Box–Behnken experimental design of Formulation-A and evaluated response parameters (n = 3). [Click here to view] |
Response 1 (R1): effect of independent variables on particle size
The R2 of the particle size was 0.9952; the adjusted R2 was 0.9890, and the predicted R2 was 0.9677, representing a reasonable agreement; that is, the difference is less than 0.2.
The Model F-value of 160.96 indicates that the model is significant (p < 0.05). In this case, A, C, AC, and C² are significant model terms.
The 3D response surface plot unveiling the influence of the independent variables on the particle size is shown in Figure 1. This study unveils the fact that with the increase in the polymer-to-drug ratio (A), the particle size of the Formulation-A was increased. This may be due to a greater concentration of the polymer in the formulation solution which may require greater force to breakdown the polymer into finer particles (Jagtap et al., 2012). On the contrary, the size of the microsphere was decreased with the increase in solvent ratio (B) (DCM: alcohol). When a good drug solubilizing solvent (alcohol) diffuses into the poor drug solubilizing solvent (DCM), it causes the precipitation of the EnM and the polymer; hence less DCM volume results in greater precipitation resulting in greater particle size (Savjani et al., 2012). Furthermore, the particle size of the microsphere decreased as the stirring time increased. This might be because of the higher shear forces responsible for the breakdown of the particle size.
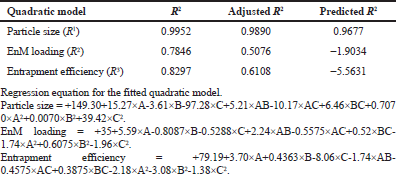 | Table 3: Summary of the results of regression analysis for responses R1, R2, and R3 .tting the quadratic models. [Click here to view] |
Out of the 17 prepared formulations, Formulation 7 exhibited the lowest particle size of 84 ± 19.97 μm, while formulation two exhibited the highest particle size of 315.21 ± 13.43 μm.
Response 2 (R2): effect of the independent variable on drug loading
The R2 of drug loading was 0.7846, and the adjusted R2 was 0.5076, representing a well fit.
The Model F-value of 2.83 indicates that the model is significant (p < 0.05). In this case, A is a significant model term.
The 3D response surface plot revealing the influence of the independent variable on drug loading is shown in Figure 2. This study unfolds that, with the increase in the polymer-to-drug ratio, there was an increase in drug loading. However, the drug loading declined with the increment in the solvent ratio. With the increase in the solvent ratio, drug loading might have been decreased due to the alcohol-induced cosolvent effect (Qiao et al., 2018). Drug loading also decreased with the increase in stirring time. However, the effect of solvent ratio and stirring time in drug loading was not significant.
From the 17 different formulations, the lowest drug loading was observed in Formulation 7 with 22.23% ± 4.17%, while Formulation 2 presented 42% ± 7.31%.
Response 3 (R3): effect of independent variables on entrapment efficiency
The R2 of drug entrapment efficiency was 0.8297, and the adjusted R2 was 0.6108, representing a well fit.
The Model F-value of 3.79 implies that the model is significant (p < 0.05). In this case, C is a significant model term.
The 3D response surface plot unveiling the influence of an independent variable on drug entrapment efficiency is shown in Figure 3. This study suggests the increment in the drug-to-polymer ratio results in an increment in entrapment efficiency. This observation might be due to the availability of a greater amount of polymers for the complete encapsulation of the EnM molecule (Thapa et al., 2018). Entrapment efficiency was increased with the increase in the solvent ratio, which might be because of a decline in the alcohol concentration in higher solvent ratio, resulting in a lower alcohol-induced cosolvent effect. However, the effect of the solvent ratio on entrapment efficiency was not significant. There was a significant decline in drug entrapment efficiency with the increase in stirring time, which may be due to prolonged exposure of the microspheres under a higher shear force that is sufficient to break the microspheres resulting in a decrease in entrapment efficiency.
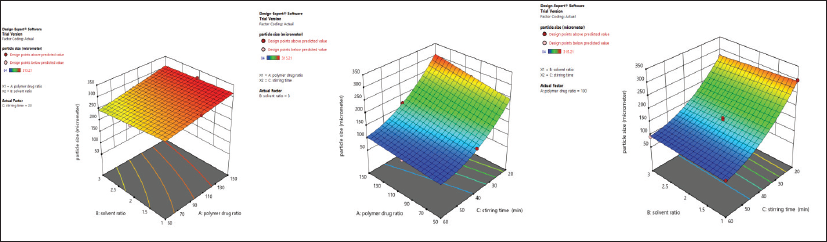 | Figure 1: 3D response surface plot unveiling the simultaneous influence of independent variables on particle size in Formulation-A. [Click here to view] |
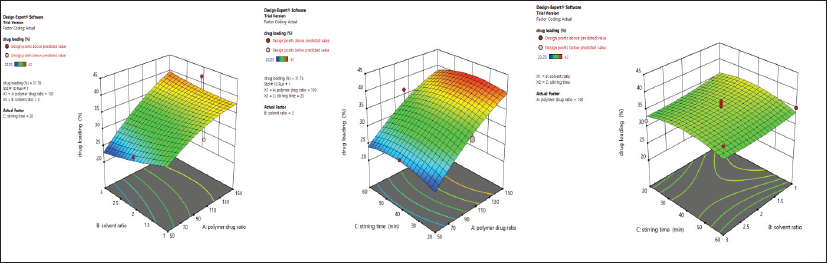 | Figure 2: 3D response surface plot unveiling simultaneous influence of independent variables on drug loading % in Formulation-A. [Click here to view] |
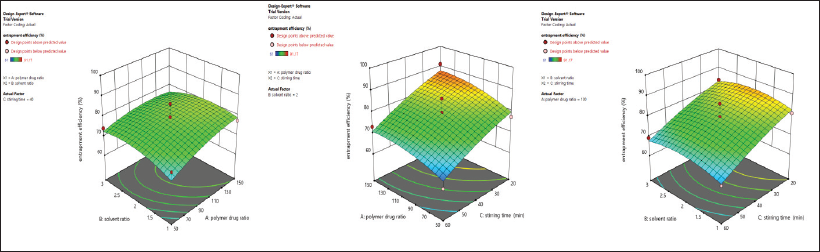 | Figure 3: 3D response surface plot unveiling the simultaneous influence of independent variables on entrapment efficiency % in Formulation-A. [Click here to view] |
From the 17 formulations, the maximum % drug entrapment efficiency was observed in Formulation 2 with 91.17% ± 3.17%, and the minimum % EnM entrapment efficiency was observed in Formulation 7 with 61 ± 1.89.
Particle size, drug loading, entrapment efficiency, and % yield of the optimized formulation
EnM-loaded optimized ethanol microsphere (Formulation-A) was selected based on minimum particle size, maximum drug loading, and entrapment efficiency. The particle size of the optimized formulation was 143.11 ± 27.75 μm, the drug loading was 37.31% ± 5.73%, and the entrapment efficiency was 76.89% ± 4.97%. The morphological shape of Formulation-A was found to be spherical and is shown in Figure 4. Similarly, the particle size, drug loading, and entrapment efficiency of Formulation-B were 158.13 ± 25.11 μm, 40.13% ± 6.12%, and 99.19% ± 1.24%, respectively. The drug encapsulation efficiency and drug loading in Formulation-A were comparatively lower than Formulation-B. It might be because of the presence of ethanol, which increases the aqueous solubility of the EnM, while in Formulation-B, drug loading and encapsulation efficiency are higher because of the absence of alcohol-induced cosolvent effect and lower solubility of EnM in acetonitrile as compared to alcohol (Qiao et al., 2018). The percentage yield of Formulation-A and Formulation-B was 34.19% and 29.314%, respectively.
Buoyancy
The buoyancy percentage of Formulation-A and Formulation-B after 12 hours was found to be 67% ± 3.82% and 61% ± 2.71%, respectively. The floating ability of Formulation-A was higher, which might be due to the presence of large air-core resulting in a decrease in density of the microsphere compared to the gastric fluid (Bhardwaj et al., 2014).
Micrometric study
Both formulations were better in terms of flowability. The angle of repose value for Formulation-A and Formulation-B was found to be 18 ± 2.75º and 23.2 ± 2.12º, while the Hausner’s ratio value was found to be 1.19 ± 0.0002 and 1.19 ± 0.0002, respectively. Similarly, Carr’s index value of Formulation-A and Formulation-B was found to be 16.25 ± 0.91 and 18.47 ± 1.002, respectively, indicating their excellent compressibility. Both the formulations were better in terms of flow properties with a low angle of repose, Carr’s index, and low Hausner’s ratio.
 | Figure 4: The morphological shape of floating microspheres observed through an Olympus BX53 research microscope (A). Formulation of EnM-loaded floating microspheres by solvent evaporation technique (B). [Click here to view] |
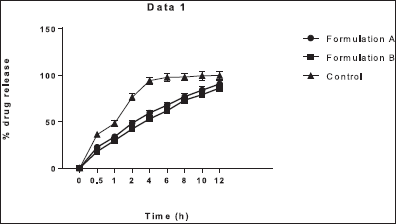 | Figure 5: In vitro drug release profile of Formulation-A, Formulation-B, and control in the acidic buffer (pH 1.2). [Click here to view] |
In vitro drug release study
It was observed that the in vitro drug release of Formulation-A was greater than Formulation-B in the simulated gastric fluid (acidic buffer, pH 1.2) as shown in Figure 5. The cumulative percentage drug release of Formulation-A and Formulation-B was 90.52% ± 4.11% and 86.23% ± 3.81%, respectively, as shown in Table 4. The greater cumulative drug release in Formulation-A was due to the use of the ethanol. As a solvent, ethanol might act as a cosolvent during the dissolution of the microsphere since the solubility of EnM is higher in alcohol compared to acetonitrile, consequently enhancing solubilization of EnM itself (Qiao et al., 2018). The in vitro drug release of Formulation-A was nonsignificantly higher than Formulation-B at 1 and 8 hours (p > 0.05). Otherwise, the in vitro EnM release of Formulation-A and Formulation-B was significantly higher than the control (p < 0.05). The polymer HPMC was used as a swelling agent and ethyl cellulose was used to increase the buoyancy of the microspheres (Kausik et al., 2015).
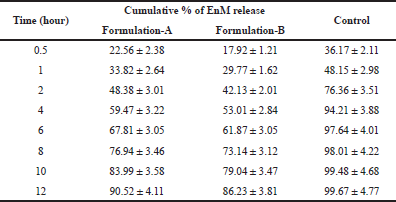 | Table 4: Cumulative % of drug release of Formulation-A and Formulation-B with time (n = 3). [Click here to view] |
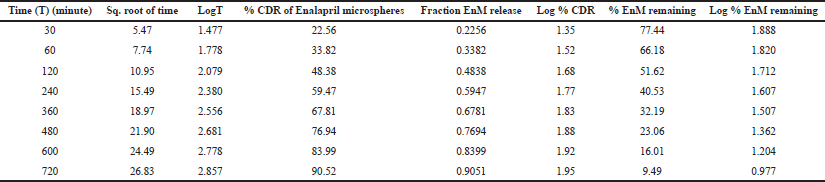 | Table 5: In vitro drug release kinetics of Formulation-A in the acidic buffer (pH 1.2). [Click here to view] |
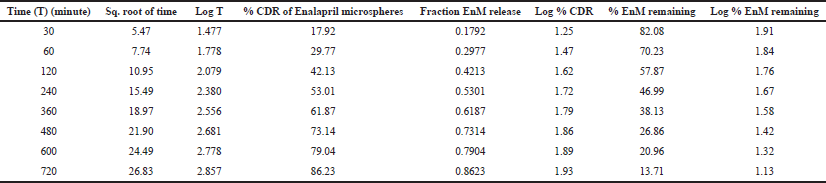 | Table 6: In vitro drug release kinetics of Formulation-B in the acidic buffer (pH 1.2). [Click here to view] |
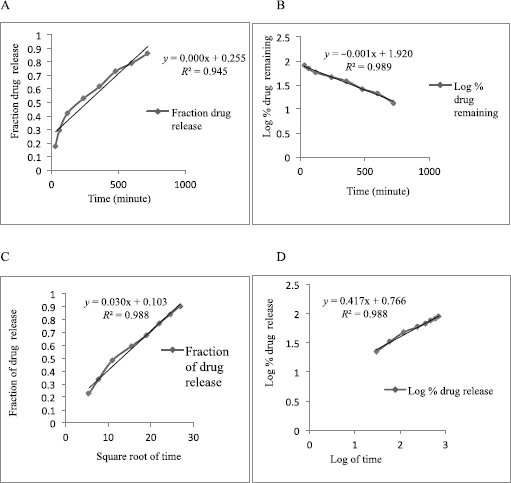 | Figure 6: Release kinetics of Formulation-A in zero-order (A), first-order (B), Higuchi model (C), and Korsmeyer–Peppas model (D) in the acidic buffer (pH 0.1). [Click here to view] |
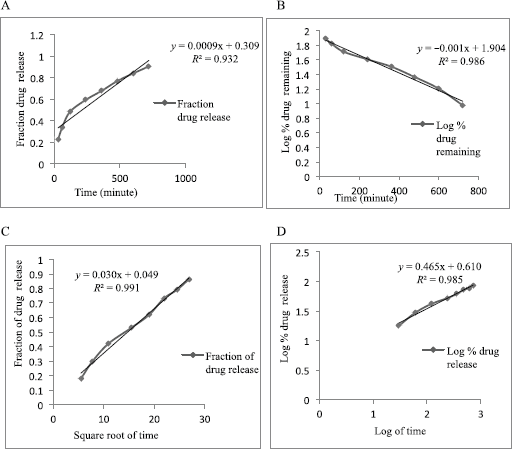 | Figure 7: Release kinetics of Formulation-B in zero-order (A), first-order (B), Higuchi model (C), and Korsmeyer–Peppas model (D) in the acidic buffer (pH 0.1). [Click here to view] |
The result of the drug release from the microsphere was fitted to the different release kinetics: “zero-order,” “first-order,” “Higuchi,” and “Korsmeyer–Peppas model”, which were taken into consideration. The in vitro drug release kinetics for both Formulation-A and Formulation-B in the acidic buffer (pH 1.2) is depicted in Tables 5 and 6, respectively. For Formulation-A, the maximum R2 value of (0.988) in the simulated gastric fluid (pH 1.2) was observed with the “Korsmeyer–Peppas model”, although the R2 value of 0.988 resembles the “Higuchi model” as well. The latter was not considered because of the less number of intersecting points compared to the Korsmeyer–Peppas model. R2 values for the “first-order model” and “zero-order model” were observed to be 0.986 and 0.932, respectively. The different release kinetics in the gastric fluid are shown in Figure 6. Similarly, for Formulation-B, the maximum R2 value of 0.985 in the simulated gastric fluid (pH 1.2) was observed with “Higuchi model”, followed by the “first-order model” (R2 = 0.989), “Korsmeyer–Peppas model” (R2 = 0.985), and “zero-order model” (R2 = 0s.956). It was observed that Formulation-B followed “Higuchi model kinetics”. The different release kinetics for Formulation-B in a gastric fluid are shown in Figure 7.
Hence, from this study, it is concluded that the EnM release from Formulation-A followed “Korsmeyer–Peppas” model kinetics. Similarly, the EnM release from Formulation-B followed the Higuchi model.
CONCLUSION
EnM-loaded floating microspheres were successfully prepared by the solvent evaporation method and optimized by the response surface method. The optimized Formulation-A exhibited particle size of 143.11 ± 27.75 μm, drug loading of 37.31% ± 5.73%, and entrapment efficiency of 76.89% ± 4.97%. The in vitro drug release of Formulation-A was higher than that of Formulation-B. Both the formulations have shown good buoyancy for more than 12 hours with a good sustained release property.
ACKNOWLEDGEMENTS
The authors sincerely acknowledge Lomus Pharmaceuticals (Kathmandu, Nepal) for kindly providing the gift sample of EnM. They also sincerely thank Dr. Anand Kumar for providing the laboratory facility to successfully carry out the research work.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No humans/animals were considered for this study.
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIALS
The data sets supporting the results of this research will be available from Chhitij Thapa, upon request.
CONFLICT OF INTEREST
There are no conflicts of interest related to the publication of this paper.
FUNDING
This study was funded by the Universal College of Medical Sciences, Siddhartha Nagar, Nepal.
REFERENCES
Abbas Z, Marihal S. Gellan gum-based mucoadhesive microspheres of almotriptan for nasal administration: formulation optimization using factorial design, characterization and in vitro evaluation. J Pharm Bioallied Sci, 2014; 6(1):269. CrossRef
Agrawal GR, Wakte P, Shelke S. Formulation, physicochemical characterization, and in vitro evaluation of human insulin-loaded microspheres as a potential oral Carrier. Prog Biomater, 2017; 6:125–36. CrossRef
Amid M, Manap Y, Azmira F, Hussin M, Sarker ZI. A novel liquid/liquid extraction process composed of surfactant and acetonitrile for purification of polygalacturonase enzyme from Durio zibethinus. J Chromatogr B Analyt Technol Biomed Life Sci, 2015; 993:1–8. CrossRef
Ammar HO, Ghorab MM, Mahmoud AA, Noshi SH. Formulation of risperidone in floating microparticles to alleviate its extrapyramidal side effects. Future J Pharm Sci, 2016; 2:43–59. CrossRef
Asija R, Sharma D, Mali KR. Development and evaluation of ethyl cellulose-based floating microspheres of atorvastatin by novel solvent evaporation matrix erosion method. Pharm Chem J, 2015; 2(1):14–24.
Beringhs AOR, Fonseca ABS, Campos AMD, Sonaglio D. Association of PLGA microspheres to carrier pellets by fluid bed coating: a novel approach towards improving the flowability of microparticles. J Pharm, 2018; 2018:1–12. CrossRef
Bhardwaj P, Chaurasia D, Singh R, Swarup A. Development and characterization of novel site-specific hollow floating microspheres bearing 5-Fu for stomach targeting. Sci World J, 2014; 2014:1–11. CrossRef
Campbell N, Young ER, Drouin D, Legowski B, Adams MA, Farrell J, Kaczorowski J, Lewanczuk R, Lum-Kwong MM, Tobe S. A framework for discussion on how to improve prevention, management, and control of hypertension in Canada. Can J Cardiol, 2012; 28:262–9. CrossRef
Chatterjee S, Salaün F, Campagne C. Development of multilayer microcapsules by a phase coacervation method based on ionic interactions for textile applications. Pharmaceutics, 2014; 6:281–97. CrossRef
Farooq U, Khan S, Nawaz S, Ranjha NM, Haider MS, Khan MM, Dar E, Nawaz A. Enhanced gastric retention and drug release via the development of novel floating microspheres based on Eudragit E100 and polycaprolactone: synthesis and in vitro evaluation. Des Monomers Polym, 2017; 20(1):419–33. CrossRef
Gao, HL, Jiang X. The progress of novel drug delivery systems. Acta Pharm Sin, 2017; 52(2):181–8.
Garg R, Gupta G. Gastro retentive floating microspheres of silymarin: preparation and in vitro evaluation. Trop J Pharm Res, 2010; 9(1):59–66. CrossRef
Gaur PK, Mishra S, Bajpai M. Formulation-And evaluation of controlled release of telmisartan microsphere: in vitro/in vivo study. J Food Drug Anal, 2014; 22:543. CrossRef
Gupta R, Prajapati SK, Pattnaik S, Bhardwaj P. Formulation and evaluation of novel stomach specific floating microspheres bearing famotidine for treatment of gastric ulcer and their radiographic study. Asian Pac J Trop Biomed, 2014; 4(9):729–35. CrossRef
Hu M, Guo J, Yu Y, Cao L, Xu Y. Research advances of microencapsulation and its prospects in the petroleum industry. Materials (Basel), 2017; 10(4):369. CrossRef
Ipate AM, Hamciue C, Kalvachev Y, Gherman S. New cryogels based on polymers and zeolite L for controlled Enalapril Maleate release. J Drug Deliv Sci Technol, 2018; 44:505–12. CrossRef
Jagtap YM, Bhujbal RK, Ranade AN, Ranpise NS. Effect of various polymers concentrations on the physicochemical properties of floating microspheres. Indian J Pharm Sci, 2012; 74(6):512–20. CrossRef
Kausik YA, Tiwari AK, Gaur A. Role of excipients and polymeric advancements in preparation of floating EnM delivery systems. Int J Pharm Investig, 2015; 5(1):01–12. CrossRef
Nila MV, Sudhir MR, Cinu TA, Aleykutty NA, Jose S. Floating microspheres of carvedilol as gastroretentive drug delivery system: 32 full factorial design and in vitro evaluation. Drug Deliv, 2014; 21(2):110–17. CrossRef
Patela K, Jainb PK, Baghelb R, Tagdea P, Patila A. Preparation and in vitro evaluation of a micro balloon delivery system for domperidone. Pharm Lett, 2011; 3:131–41.
Prajapati CV, Patel RP, Prajapati BG. Formulation, optimization, and evaluation of sustained-release microsphere of ketoprofen. J Pharm Bioallied Sci, 2012; 4(1):101–3 CrossRef
Qiao B, Du C, Dong R, Jin Z, Zhang Y, Ye T, Roth M. Experiment and computation of solubility and dissolution properties for Enalapril Maleate and its intermediate in pure solvents. J Chem Eng Data, 2018; 63(12):4306–13. CrossRef
Ramteke KH, Jadhav VB, Kulkarni NS, Kharat AR, Diwate SB. Preparation, evaluation, and optimization of multiparticulate system of mebendazole for colon targeted drug delivery by using natural polysaccharides. Adv Pharm Bull, 2015; 5(3):361–71. CrossRef
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm, 2012; 2012:1–10. CrossRef
Senthikumar S, Jaykar B, Kavimani S. Formulation, characterization and in vitro evaluation of floating microsphere containing rabeprazole sodium. Int J Ther Pharm Sci, 2010; 1(6):274–82.
Sharma M, Kohli S, Dinda A. In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer. Saudi Pharm J, 2015; 23:675–82. CrossRef
Subham B, Gaurav C, Dilipkumar P, Gosh AK. Investigation on crosslinking density for development of novel interpenetrating polymer network (IPN) based formulation. J Sci Ind Res, 2010; 69:777–84.
Swamy BY, Prasad CV, Prabhakar MN, Rao KC, Subha MCS, Chung I. Biodegradable chitosan-g-poly(methacrylamide) microspheres for controlled release of hypertensive drug. J Polym Environ, 2013; 21:1128–34. CrossRef
Tamizharasi S, Sivakumar T, Rathi JC. Preparation and evaluation of aceclofenac floating oral delivery system. Pharm Sin, 2011; 2:43–53. CrossRef
Thabet Y, Walsh J, Breitkreutz J. Flexible and precise dosing of Enalapril Maleate for all pediatric age groups utilizing orodispersible minitablets. Int J Pharm, 2018; 541:136–42. CrossRef
Thapa C, Ahad A, Aqil M, Imam SS, Sultana Y. Formulation and optimization of nanostructured lipid carriers to enhance the oral bioavailability of telmisartan using Box–Behnken design. J Drug Deliv Sci Technol, 2018; 44:431–9. CrossRef
Vaidya A, Pathak RP, Pandey MR. Prevalence of hypertension in the nepalese community triples in a year: a repeat cross-sectional study in rural Kathmandu. Indian Heart J, 2012; 6402:128–31. CrossRef
Wang W, Zhang J, Yang S, Zhang H, Yang H, Yue G. Experimental study on the angle of repose of pulverized coal. Particuology, 2010; 8(5):482–6. CrossRef
Wen H, Jung H, Li X. Drug delivery approaches in addressing clinical pharmacology- related issues: opportunities and challenges. AAPS J, 2015; 17(6):1327–40. CrossRef
Zhang W, Chen L, Zhe A, Liu G, Zhang L, Wang Y, Shao Y, Liu Y. Gastro retentive floating microspheres of carvedilol: preparation, in vitro, and in vivo characterization. J Biomater Tissue Eng, 2016; 6:74–8. CrossRef